Abstract
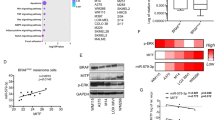
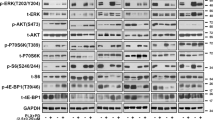
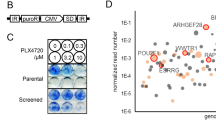
While combination BRAF/MEK inhibition has improved survival in BRAFV600 mutant melanoma, targeted therapies for BRAFWT melanoma remain limited. Microphthalmia transcription factor (MITF), a lineage-specific transcription factor that regulates melanocyte proliferation and melanin synthesis, represents a promising melanoma-specific drug target. In this study, we evaluated TT-012, a recently identified MITF dimerization specific inhibitor, and surprisingly found that most BRAFWT melanoma lines were resistant to TT-012 due to low MITF transcriptional activity and reduced dependency on MITF for proliferation. High-throughput drug screen identified tivozanib, an FDA-approved drug targeting VEGFR and other receptor tyrosine kinases (RTKs), which sensitized cells to TT-012. Mechanistically, tivozanib induced cell state transition from MITFlow to MITFhigh state via VEGFR2 inhibition followed by NF-κB pathway activation, restoring MITF transcriptional activity and growth dependency. The combination of tivozanib and TT-012 synergistically inhibited melanoma growth both in vitro and in vivo, underscoring its potential as a novel therapeutic strategy for BRAFWT melanoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30–9.
Robert C, Grob JJ, Stroyakovskiy D, Karaszewska B, Hauschild A, Levchenko E, et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med. 2019;381:626–36.
Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet. 2015;386:444–51.
Hayward NK, Wilmott JS, Waddell N, Johansson PA, Field MA, Nones K, et al. Whole-genome landscapes of major melanoma subtypes. Nature. 2017;545:175–80.
Ascierto PA, Long GV, Robert C, Brady B, Dutriaux C, Di Giacomo AM, et al. Survival outcomes in patients with previously untreated BRAF wild-type advanced melanoma treated with nivolumab therapy: three-year follow-up of a randomized phase 3 trial. JAMA Oncol. 2019;5:187–94.
Johnpulle RA, Johnson DB, Sosman JA. Molecular targeted therapy approaches for BRAF wild-type melanoma. Curr Oncol Rep. 2016;18:6.
Massa RC, Kirkwood JM. Targeting the MAPK pathway in advanced BRAF wild-type melanoma. Ann Oncol. 2019;30:503–5.
Goding CR. Commentary. A picture of Mitf in melanoma immortality. Oncogene. 2011;30:2304–6.
Goding CR, Arnheiter H. MITF-the first 25 years. Genes Dev. 2019;33:983–1007.
Garraway LA, Widlund HR, Rubin MA, Getz G, Berger AJ, Ramaswamy S, et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature. 2005;436:117–22.
Potrony M, Puig-Butille JA, Aguilera P, Badenas C, Tell-Marti G, Carrera C, et al. Prevalence of MITF p.E318K in patients with melanoma independent of the presence of CDKN2A causative mutations. JAMA Dermatol. 2016;152:405–12.
Chauhan JS, Holzel M, Lambert JP, Buffa FM, Goding CR. The MITF regulatory network in melanoma. Pigment Cell Melanoma Res. 2022;35:517–33.
Goyal Y, Busch GT, Pillai M, Li J, Boe RH, Grody EI, et al. Diverse clonal fates emerge upon drug treatment of homogeneous cancer cells. Nature. 2023;620:651–9.
Boumahdi S, de Sauvage FJ. The great escape: tumour cell plasticity in resistance to targeted therapy. Nat Rev Drug Discov. 2020;19:39–56.
Bai X, Fisher DE, Flaherty KT. Cell-state dynamics and therapeutic resistance in melanoma from the perspective of MITF and IFNgamma pathways. Nat Rev Clin Oncol. 2019;16:549–62.
Rambow F, Marine JC, Goding CR. Melanoma plasticity and phenotypic diversity: therapeutic barriers and opportunities. Genes Dev. 2019;33:1295–318.
Carotenuto P, Romano A, Barbato A, Quadrano P, Brillante S, Volpe M, et al. Targeting the MITF/APAF-1 axis as salvage therapy for MAPK inhibitors in resistant melanoma. Cell Rep. 2022;41:111601.
Tsoi J, Robert L, Paraiso K, Galvan C, Sheu KM, Lay J, et al. Multi-stage differentiation defines melanoma subtypes with differential vulnerability to drug-induced iron-dependent oxidative stress. Cancer Cell. 2018;33:890–904.e5.
Smith MP, Brunton H, Rowling EJ, Ferguson J, Arozarena I, Miskolczi Z, et al. Inhibiting drivers of non-mutational drug tolerance is a salvage strategy for targeted melanoma therapy. Cancer Cell. 2016;29:270–84.
Liu Z, Chen K, Dai J, Xu P, Sun W, Liu W, et al. A unique hyperdynamic dimer interface permits small molecule perturbation of the melanoma oncoprotein MITF for melanoma therapy. Cell Res. 2023;33:55–70.
Boyle H. Tivozanib: a novel VGFR inhibitor for kidney cancer. Transl Androl Urol. 2013;2:114–6.
Masone MC. Tivozanib monotherapy outperforms combination therapy in post-ICI RCC. Nat Rev Urol. 2024;21:706.
Chang E, Weinstock C, Zhang L, Fiero MH, Zhao M, Zahalka E, et al. FDA approval summary: tivozanib for relapsed or refractory renal cell carcinoma. Clin Cancer Res. 2022;28:441–5.
Korsunsky I, Millard N, Fan J, Slowikowski K, Zhang F, Wei K, et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat Methods. 2019;16:1289–96.
Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM, et al. Comprehensive integration of single-cell data. Cell. 2019;177:1888–902.e21.
Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–60.
Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, Sougnez C, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol. 2013;31:213–9.
Zheng S, Wang W, Aldahdooh J, Malyutina A, Shadbahr T, Tanoli Z, et al. SynergyFinder Plus: toward better interpretation and annotation of drug combination screening datasets. Genomics Proteomics Bioinformatics. 2022;20:587–96.
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–9.
Luo W, Friedman MS, Shedden K, Hankenson KD, Woolf PJ. GAGE: generally applicable gene set enrichment for pathway analysis. BMC Bioinformatics. 2009;10:161.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50.
Lomazzi M, Moroni MC, Jensen MR, Frittoli E, Helin K. Suppression of the p53- or pRB-mediated G1 checkpoint is required for E2F-induced S-phase entry. Nat Genet. 2002;31:190–4.
Hallstrom TC, Mori S, Nevins JR. An E2F1-dependent gene expression program that determines the balance between proliferation and cell death. Cancer Cell. 2008;13:11–22.
Yadav B, Wennerberg K, Aittokallio T, Tang J. Searching for drug synergy in complex dose–response landscapes using an interaction potency model. Comput Struct Biotechnol J. 2015;13:504–13.
Nakamura K, Taguchi E, Miura T, Yamamoto A, Takahashi K, Bichat F, et al. KRN951, a highly potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, has antitumor activities and affects functional vascular properties. Cancer Res. 2006;66:9134–42.
Hartman ML, Czyz M. MITF in melanoma: mechanisms behind its expression and activity. Cell Mol Life Sci. 2015;72:1249–60.
Saha B, Singh SK, Sarkar C, Bera R, Ratha J, Tobin DJ, et al. Activation of the Mitf promoter by lipid-stimulated activation of p38-stress signalling to CREB. Pigment Cell Res. 2006;19:595–605.
Goodall J, Carreira S, Denat L, Kobi D, Davidson I, Nuciforo P, et al. Brn-2 represses microphthalmia-associated transcription factor expression and marks a distinct subpopulation of microphthalmia-associated transcription factor-negative melanoma cells. Cancer Res. 2008;68:7788–94.
Smith MP, Sanchez-Laorden B, O’Brien K, Brunton H, Ferguson J, Young H, et al. The immune microenvironment confers resistance to MAPK pathway inhibitors through macrophage-derived TNFalpha. Cancer Discov. 2014;4:1214–29.
Levy C, Khaled M, Fisher DE. MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol Med. 2006;12:406–14.
Rauluseviciute I, Riudavets-Puig R, Blanc-Mathieu R, Castro-Mondragon JA, Ferenc K, Kumar V, et al. JASPAR 2024: 20th anniversary of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2024;52:D174–D82.
Croft M, Salek-Ardakani S, Ware CF. Targeting the TNF and TNFR superfamilies in autoimmune disease and cancer. Nat Rev Drug Discov. 2024;23:939–61.
Takada H, Sasagawa Y, Yoshimura M, Tanaka K, Iwayama Y, Hayashi T, et al. Single-cell transcriptomics uncovers EGFR signaling-mediated gastric progenitor cell differentiation in stomach homeostasis. Nat Commun. 2023;14:3750.
Miller I, Min M, Yang C, Tian C, Gookin S, Carter D, et al. Ki67 is a graded rather than a binary marker of proliferation versus quiescence. Cell Rep. 2018;24:1105–12.e5.
Long GV, Hauschild A, Santinami M, Kirkwood JM, Atkinson V, Mandala M, et al. Final results for adjuvant dabrafenib plus trametinib in stage III melanoma. N Engl J Med. 2024;391:1709–20.
Wellbrock C, Arozarena I. Microphthalmia-associated transcription factor in melanoma development and MAP-kinase pathway targeted therapy. Pigment Cell Melanoma Res. 2015;28:390–406.
Landsberg J, Kohlmeyer J, Renn M, Bald T, Rogava M, Cron M, et al. Melanomas resist T-cell therapy through inflammation-induced reversible dedifferentiation. Nature. 2012;490:412–6.
Konieczkowski DJ, Johannessen CM, Abudayyeh O, Kim JW, Cooper ZA, Piris A, et al. A melanoma cell state distinction influences sensitivity to MAPK pathway inhibitors. Cancer Discov. 2014;4:816–27.
Acknowledgements
This research was funded by the the Science and Technology Commission of Shanghai Municipality (No. YDZX20223100003007 to Wei Guo), SHIPM-mu fund (No. JC202001 to Xu-hui Ma) from Shanghai Institute of Precision Medicine, Ninth People’s Hospital Shanghai Jiao Tong University School of Medicine, National Natural Science Foundation of China (No. 82204421 to Han-lin Zeng), the SHIPM-pi fund (XK2020015 to Han-lin Zeng) from the Shanghai Institute of Precision Medicine, the Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine. This work was also supported by the Class IV Peak Disciplines (Shanghai Institute of Precision Medicine), and the Innovative Research Team of High-Level Local Universities (SHSMU-ZLCX20211700) from the Shanghai Municipal Education Commission. We gratefully acknowledge the TT-012 and TYR-luciferase plasmid provided by Dr. Wang Jing from Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences. We thank Hong Lu, Shu-fang He, Jie Huang, and all staff members of the Bioimaging Facility, and Proteomics Platform at the Shanghai Institute of Precision Medicine for providing technical support and assistance in data collection.
Author information
Authors and Affiliations
Contributions
HLZ, YNM, XHM, ML designed research; XHM, YZ, XYC, YJZ, WG, MJ contributed clinical samples; YNM, MLH, XYC, YNC, SNZ performed experiments; RXL, JY, MLH analyzed data; WG, XHM, HLZ acquired funding; YZ, MJ provided resources; HLZ, YNM wrote the paper; HLZ, XHM, WG, MJ supervised the research. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, Yn., Ma, Xh., Hao, Ml. et al. Receptor tyrosine kinase inhibitor tivozanib regulates cell state plasticity and restores MITF dependency in BRAF wild-type melanoma. Acta Pharmacol Sin (2025). https://doi.org/10.1038/s41401-025-01599-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41401-025-01599-3