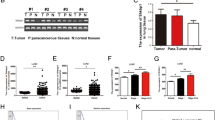
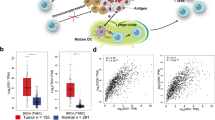
Abstract
Triple-negative breast cancer (TNBC) is highly prone to lung metastasis, primarily driven by epithelial-mesenchymal transition (EMT) and vasculogenic mimicry (VM). Therefore, inhibiting EMT and VM represents a promising therapeutic strategy for TNBC. The immunosuppressive tumor microenvironment contributes substantially to poor treatment outcomes, with M2-type macrophages secreting excessive levels of TGF-β that promote both EMT and VM. In this study, we proposed a combination therapy strategy involving shikonin (SHK) and JQ1 delivered via a mesoporous polydopamine-based Pickering emulsion (termed MPDA@PE). This formulation significantly suppressed tumor growth and lung metastasis by inducing apoptosis in TNBC and inhibiting TGF-β-induced EMT and VM. Furthermore, MPDA@PE can be incorporated into a thermosensitive hydrogel for application in the prevention of TNBC recurrence and lung metastasis following surgical resection. These findings highlight a potential therapeutic approach for effective TNBC treatment.

The combined administration of SHK and JQ1 inhibits both EMT and VM. This approach disrupts the nutrient supply in tumor tissues by blocking VM and suppresses tumor metastasis through EMT inhibition. Consequently, it demonstrates therapeutic efficacy against TNBC recurrence post-surgery and effectively limits lung metastasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13:674–90.
Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–48.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15.
Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–92.
Liu S, Kang M, Ren Y, Zhang Y, Ba Y, Deng J, et al. The interaction between vasculogenic mimicry and the immune system: mechanistic insights and dual exploration in cancer therapy. Cell Prolif. 2025:e13814.
Cheng B, Xie M, Zhou Y, Li T, Liu W, Yu W, et al. Vascular mimicry induced by m6A mediated IGFL2-AS1/AR axis contributes to pazopanib resistance in clear cell renal cell carcinoma. Cell Death Discov. 2023;9:121.
Wang J, Chen Z, Zhao P, Wang Y, Chen J, Lin Q. PDGFR-alpha shRNA-polyplex for uveal melanoma treatment via EMT mediated vasculogenic mimicry interfering. J Nanobiotechnol. 2024;22:797.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90.
Wei X, Ge Y, Zheng Y, Zhao S, Zhou Y, Chang Y, et al. Hybrid EMT phenotype and cell membrane tension promote colorectal cancer resistance to ferroptosis. Adv Sci. 2025;12:e2413882.
Chang TM, Fang WY, Hsu HP, Chu PY, Jiang SS, Huang KW, et al. PCK2 promotes invasion and epithelial-to-mesenchymal transition in triple-negative breast cancer by promoting TGF-beta/SMAD3 signaling through inhibiting TRIM67-mediated SMAD3 ubiquitination. Cancer Biol Ther. 2025;26:2478670.
Zhang X, Zhang J, Zhou H, Liu G, Li Q. Rho kinase mediates transforming growth factor-beta1-induced vasculogenic mimicry formation: involvement of the epithelial-mesenchymal transition and cancer stemness activity. Acta Biochim Biophys Sin. 2020;52:411–20.
Zhao X, Zhu Y, Hu J, Jiang L, Li L, Jia S, et al. Shikonin inhibits tumor growth in mice by suppressing pyruvate kinase M2-mediated aerobic glycolysis. Sci Rep. 2018;8:14517.
Huang Y. Targeting glycolysis for cancer therapy using drug delivery systems. J Control Rel. 2023;353:650–62.
Li X, Zeng X. Shikonin suppresses progression and epithelial-mesenchymal transition in hepatocellular carcinoma (HCC) cells by modulating miR-106b/SMAD7/TGF-beta signaling pathway. Cell Biol Int. 2020;44:467–76.
Long L, Xiong W, Lin F, Hou J, Chen G, Peng T, et al. Regulating lactate-related immunometabolism and EMT reversal for colorectal cancer liver metastases using shikonin targeted delivery. J Exp Clin Cancer Res. 2023;42:117.
Qiu N, Liu Y, Liu Q, Chen Y, Shen L, Hu M, et al. Celastrol nanoemulsion induces immunogenicity and downregulates PD-L1 to boost abscopal effect in melanoma therapy. Biomaterials. 2021;269:120604.
da Motta LL, Ledaki I, Purshouse K, Haider S, De Bastiani MA, Baban D, et al. The BET inhibitor JQ1 selectively impairs tumour response to hypoxia and downregulates CA9 and angiogenesis in triple negative breast cancer. Oncogene. 2017;36:122–32.
Wang H, Tang Y, Fang Y, Zhang M, Wang H, He Z, et al. Reprogramming tumor immune microenvironment (TIME) and metabolism via biomimetic targeting codelivery of Shikonin/JQ1. Nano Lett. 2019;19:2935–44.
Bagratuni T, Mavrianou N, Gavalas NG, Tzannis K, Arapinis C, Liontos M, et al. JQ1 inhibits tumour growth in combination with cisplatin and suppresses JAK/STAT signalling pathway in ovarian cancer. Eur J Cancer. 2020;126:125–35.
Song S, Liu L, Yu Y, Zhang R, Li Y, Cao W, et al. Inhibition of BRD4 attenuates transverse aortic constriction- and TGF-beta-induced endothelial-mesenchymal transition and cardiac fibrosis. J Mol Cell Cardiol. 2019;127:83–96.
Zhuo M, Yuan C, Han T, Hu H, Cui J, Jiao F, et al. JQ1 effectively inhibits vasculogenic mimicry of pancreatic ductal adenocarcinoma cells via the ERK1/2-MMP-2/9 signaling pathway both in vitro and in vivo. Am J Transl Res. 2019;11:1030–9.
Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q, et al. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020;48:W509–W14.
Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704.
Perez-Tomas R, Perez-Guillen I. Lactate in the tumor microenvironment: an essential molecule in cancer progression and treatment. Cancers. 2020;12:3244.
Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9.
Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72.
Wang C, Zhang M, Peng J, Zhang M, Lu C, Qi X, et al. Combining cisplatin with Pinellia pedatisecta Schott lipid-soluble extract induces tumor immunogenic cell death in cervical cancer. Phytomedicine. 2024;128:155504.
Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61.
Yang J, Lu Y, Lin YY, Zheng ZY, Fang JH, He S, et al. Vascular mimicry formation is promoted by paracrine TGF-beta and SDF1 of cancer-associated fibroblasts and inhibited by miR-101 in hepatocellular carcinoma. Cancer Lett. 2016;383:18–27.
Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–96.
Gradishar WJ, Moran MS, Abraham J, Abramson V, Aft R, Agnese D, et al. NCCN guidelines(R) insights: breast cancer, version 4.2023. J Natl Compr Canc Netw. 2023;21:594–608.
Pantel K, Alix-Panabieres C. Liquid biopsy and minimal residual disease - latest advances and implications for cure. Nat Rev Clin Oncol. 2019;16:409–24.
Belgiovine C, D’Incalci M, Allavena P, Frapolli R. Tumor-associated macrophages and anti-tumor therapies: complex links. Cell Mol Life Sci. 2016;73:2411–24.
Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61.
Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–7.
Acknowledgements
We are grateful for the support from the National Key Research and Development Program of China (2024YFA1210200, China), National Natural Science Foundation of China (82341232), Natural Science Foundation of Shanghai Municipal (24ZR1477500), High-level Innovative Research Institute (2021B0909050003) from Department of Science and Technology of Guangdong Province, and Zhongshan Municipal Bureau of Science and Technology (LJ2021001 & CXTD2022011). We thank the staff members of the Large-scale Protein Preparation System (https://cstr.cn/31129.02.NFPS.LSPS) at the National Facility for Protein Science in Shanghai (https://cstr.cn/31129.02.NFPS), for providing technical support and assistance in data collection and analysis in transmission electron microscopy and fluorescence microscope.
Author information
Authors and Affiliations
Contributions
XYX: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. DRK: Writing – original draft, Methodology, Investigation, Data curation. YY: Methodology, Investigation. LXY: Methodology, Investigation. ZWG: Methodology, Investigation. XYJ: Methodology, Investigation. HYW: Methodology, Conceptualization. YZH: Writing – review & editing, Writing – original draft, Supervision, Project administration, Funding acquisition, Formal analysis, Conceptualization.
Corresponding authors
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, Xy., Kalambhe, D.R., Yu, Y. et al. Co-delivery of shikonin and JQ1 inhibits triple-negative breast tumor progression and lung metastasis through inhibition of epithelial-mesenchymal transition and vasculogenic mimicry. Acta Pharmacol Sin 46, 3314–3326 (2025). https://doi.org/10.1038/s41401-025-01605-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-025-01605-8