Abstract
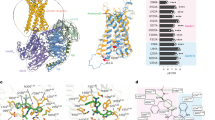
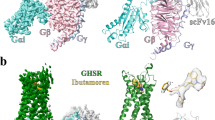
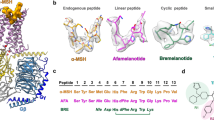
The growth hormone secretagogue receptor (GHSR) plays a critical role in regulating growth hormone release and metabolic homeostasis. Understanding the molecular mechanisms of ligand-GHSR recognition is essential for developing therapeutic interventions. In this study, we investigated the molecular recognition mechanisms of two clinically approved drugs: Macimorelin (used for diagnosing adult growth hormone deficiency) and Anamorelin (approved in Japan for cancer cachexia). Using high-resolution cryo-electron microscopy, we determined the structures of GHSR bound to Macimorelin and Anamorelin in complex with Gq proteins at resolutions of 2.63 Å and 2.52 Å, respectively. We revealed that both drugs occupied a bifurcated binding pocket divided by a conserved salt bridge between E1243.33 and R2836.55. Through systematic mutagenesis and functional studies, we identified the key residues underlying the higher binding affinity of Anamorelin compared to Macimorelin. In addition, structural comparison of GHSR in complex with different G protein subtypes elucidated the mechanisms driving G protein selectivity. Our results provide crucial insights into GHSR-drug interactions and offer valuable guidance for designing more selective and potent GHSR agonists.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci USA. 2004;101:4679–84.
Date Y, Nakazato M, Hashiguchi S, Dezaki K, Mondal MS, Hosoda H, et al. Ghrelin is present in pancreatic α-cells of humans and rats and stimulates insulin secretion. Diabetes. 2002;51:124–9.
Hosoda H, Kojima M, Kangawa K. Biological, physiological, and pharmacological aspects of ghrelin. J Pharm Sci. 2006;100:398–410.
Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–7.
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60.
Lv Y, Liang T, Wang G, Li Z. Ghrelin, a gastrointestinal hormone, regulates energy balance and lipid metabolism. Biosci Rep. 2018;38:BSR20181061.
Gahete MD, Córdoba-Chacón J, Kineman RD, Luque RM, Castaño JP. Role of ghrelin system in neuroprotection and cognitive functions: implications in Alzheimer’s disease. Peptides. 2011;32:2225–8.
Beiras-Fernandez A, Kreth S, Weis F, Ledderose C, Pöttinger T, Dieguez C, et al. Altered myocardial expression of ghrelin and its receptor (GHSR-1a) in patients with severe heart failure. Peptides. 2010;31:2222–8.
Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, et al. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002;87:2988–91.
Baessler A, Hasinoff MJ, Fischer M, Reinhard W, Sonnenberg GE, Olivier M, et al. Genetic linkage and association of the growth hormone secretagogue receptor (ghrelin receptor) gene in human obesity. Diabetes. 2005;54:259–67.
Xiao X, Tang T, Bi M, Liu J, Liu M, Jiao Q, et al. GHSR deficiency exacerbates Parkinson’s disease pathology by impairing autophagy. Redox Biol. 2024;76:103322.
Baessler A, Kwitek AE, Fischer M, Koehler M, Reinhard W, Erdmann J, et al. Association of the Ghrelin receptor gene region with left ventricular hypertrophy in the general population: results of the MONICA/KORA Augsburg Echocardiographic Substudy. Hypertension. 2006;47:920–7.
Smith RG, Cheng K, Pong S-S, Leonard R, Cohen CJ, Arena JP, et al. Mechanism of Action of GHRP-6 and Nonpeptidyl Growth Hormone Secretagogues. In: Bercu BB,Walker RF, Editors. Growth Hormone Secretagogues. (Springer New York, New York, NY, 1996), p 147–63.
Esposito A, Criscitiello C, Gelao L, Pravettoni G, Locatelli M, Minchella I, et al. Mechanisms of anorexia-cachexia syndrome and rational for treatment with selective ghrelin receptor agonist. Cancer Treat Rev. 2015;41:793–7.
Hedegaard MA, Holst B. The complex signaling pathways of the ghrelin receptor. Endocrinology. 2020;161:bqaa020.
MACRILEN. Prescribing Information. Aeterna Zentaris GmbH; 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205598s000lbl.pdf.
GHRYVELIN. Summary of Product Characteristics. Aeterna Zentaris GmbH; 2019. https://www.ema.europa.eu/en/documents/product-information/ghryvelin-epar-product-information_en.pdf.
Anamorelin Hydrochloride. Review Report. Ono Pharmaceutical Co., Ltd.; 2019. https://www.pmda.go.jp/files/000242929.pdf.
Guerlavais V, Boeglin D, Mousseaux D, Oiry C, Heitz A, Deghenghi R, et al. New active series of growth hormone secretagogues. J Med Chem. 2003;46:1191–203.
Broglio F, Boutignon F, Benso A, Gottero C, Prodam F, Arvat E, et al. EP1572: a novel peptido-mimetic GH secretagogue with potent and selective GH-releasing activity in man. J Endocrinol Invest. 2002;25:RC26–RC8.
Boer HD, Blok G-J, Veen EAVD. Clinical aspects of growth hormone deficiency in adults. Endocr Rev. 1995;16:63–86.
Maghnie M, Aimaretti G, Bellone S, Bona G, Bellone J, Baldelli R, et al. Diagnosis of GH deficiency in the transition period: accuracy of insulin tolerance test and insulin-like growth factor-I measurement. Eur J Endocrinol. 2005;152:589–96.
Hoeck HC, Vestergaard P, Jakobsen PE, Laurberg P. Test of growth hormone secretion in adults: poor reproducibility of the insulin tolerance test. Eur J Endocrinol. 1995;133:305–12.
Garcia JM, Swerdloff R, Wang C, Kyle M, Kipnes M, Biller B, et al. Macimorelin (AEZS-130)-stimulated growth hormone (GH) test: validation of a novel oral stimulation test for the diagnosis of adult GH deficiency. J Clin Endocrinol Metab. 2013;98:2422–9.
Garcia JM, Biller BM, Korbonits M, Popovic V, Luger A, Strasburger CJ, et al. Macimorelin as a diagnostic test for adult GH deficiency. J Clin Endocrinol Metab. 2018;103:3083–93.
Garcia JM, Biller BM, Korbonits M, Popovic V, Luger A, Strasburger CJ, et al. Sensitivity and specificity of the macimorelin test for diagnosis of AGHD. Endocr Connect. 2021;10:76–83.
Csákváry V, Ammer N, Bagci EB, Bolshova OV, Damholt BB, Katanic D, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of macimorelin in children with suspected growth hormone deficiency: an open-label, group comparison, dose-escalation trial. Horm Res Paediatr. 2021;94:239–50.
Garcia JM, Polvino WJ. Pharmacodynamic hormonal effects of anamorelin, a novel oral ghrelin mimetic and growth hormone secretagogue in healthy volunteers. Growth Horm IGF Res. 2009;19:267–73.
Garcia JM, Boccia RV, Graham CD, Yan Y, Duus EM, Allen S, et al. Anamorelin for patients with cancer cachexia: an integrated analysis of two phase 2, randomised, placebo-controlled, double-blind trials. Lancet Oncol. 2015;16:108–16.
Temel JS, Abernethy AP, Currow DC, Friend J, Duus EM, Yan Y, et al. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol. 2016;17:519–31.
Currow D, Temel J, Abernethy A, Milanowski J, Friend J, Fearon K. ROMANA 3: a phase 3 safety extension study of anamorelin in advanced non-small-cell lung cancer (NSCLC) patients with cachexia. Ann Oncol. 2017;28:1949–56.
Argilés JM, Busquets S, Stemmler B, López-Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer. 2014;14:754–62.
Rivas AS, Álvarez YE, Cordellat AB, Tarruella MM, Mata KM, de la Cámara MM, et al. SEOM clinical guidelines for cancer anorexia-cachexia syndrome. Clin Transl Oncol 2023;2024:1–11.
Takayama K, Kojima A, Honda C, Nakayama M, Kanemata S, Endo T, et al. Real‐world safety and effectiveness of anamorelin for cancer cachexia: Interim analysis of post‐marketing surveillance in Japan. Cancer Med. 2024;13:e7170.
Wang Y, Guo S, Zhuang Y, Yun Y, Xu P, He X, et al. Molecular recognition of an acyl-peptide hormone and activation of ghrelin receptor. Nat Commun. 2021;12:5064.
Liu H, Sun D, Myasnikov A, Damian M, Baneres J-L, Sun J, et al. Structural basis of human ghrelin receptor signaling by ghrelin and the synthetic agonist ibutamoren. Nat Commun. 2021;12:6410.
Shiimura Y, Horita S, Hamamoto A, Asada H, Hirata K, Tanaka M, et al. Structure of an antagonist-bound ghrelin receptor reveals possible ghrelin recognition mode. Nat Commun. 2020;11:4160.
Qin J, Cai Y, Xu Z, Ming Q, Ji S-Y, Wu C, et al. Molecular mechanism of agonism and inverse agonism in ghrelin receptor. Nat Commun. 2022;13:300.
Koehl A, Hu H, Maeda S, Zhang Y, Qu Q, Paggi JM, et al. Structure of the µ-opioid receptor–Gi protein complex. Nature. 2018;558:547–52.
Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, et al. Crystal structure of the β2 adrenergic receptor–Gs protein complex. Nature. 2011;477:549–55.
Paul BJ, Littler BJ, Jos F, Vogt PF, Pines SH. A practical synthesis of the pseudotripeptide RC-1291. Org Process Res Dev 2006;10:339–45.
Zheng SQ, Palovcak E, Armache J-P, Verba KA, Cheng Y, Agard DA. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat Methods. 2017;14:331–2.
Punjani A, Rubinstein JL, Fleet DJ, Brubaker MA. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat Methods. 2017;14:290–6.
Bepler T, Kelley K, Noble AJ, Berger B. Topaz-Denoise: general deep denoising models for cryoEM and cryoET. Nat Commun. 2020;11:5208.
Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–32.
Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–21.
Pettersen EF, Goddard TD, Huang CC, Meng EC, Couch GS, Croll TI, et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 2021;30:70–82.
Leyris J-P, Roux T, Trinquet E, Verdié P, Fehrentz J-A, Oueslati N, et al. Homogeneous time-resolved fluorescence-based assay to screen for ligands targeting the growth hormone secretagogue receptor type 1a. Anal Biochem. 2011;408:253–62.
Acknowledgements
The cryo-electron microscopy data were collected at the Shanghai Advanced Center for Electron Microscopy, Shanghai Institute of Materia Medica, Chinese Academy of Sciences. We thank Wen Hu for performing data collection. This work was supported by the National Natural Science Foundation of China (32171187 to YJ, 32130022 to HEX, 82121005 to XX, and HEX, 82330113 to XX, 82304579 to SMG); the National Key R&D Program of China (2022YFC2703105 to HEX); CAS Strategic Priority Research Program (XDB37030103 to HEX); Shanghai Municipal Science and Technology Major Project (2019SHZDZX02 to HEX); State Key Laboratory of Drug Research, Grant No. SKLDR-2024-TT-02 (XX and HL); the Lingang Laboratory, Grant No. LG-GG-202204-01 (HEX); the Special Research Assistant Project of Chinese Academy of Sciences (to YW).
Author information
Authors and Affiliations
Contributions
YW, HEX, and XX initiated the project. RLW expressed, optimized and purified the receptor complex. HL performed cryo-EM grid preparation, data acquisition, structure determination and model building. WH performed cryo-EM data acquisition. JS and SMG performed cell-based binding and functional assays under the supervision of XX. YZ synthesized Macimorelin and Anamorelin under the supervision of JW and HL. YW and RLW prepared the figures and wrote the draft; YWZ and YJ participated in project guidance. YW, HEX, and XX wrote the manuscript with inputs from the authors.
Corresponding authors
Ethics declarations
Competing interests
HEX is a founder of Cascade Pharmaceutics. All the other authors declare no other competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Rl., Sun, J., Liu, H. et al. Molecular recognition of two approved drugs Macimorelin and Anamorelin by the growth hormone secretagogue receptor. Acta Pharmacol Sin (2025). https://doi.org/10.1038/s41401-025-01606-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41401-025-01606-7