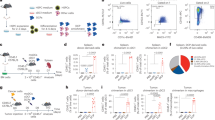
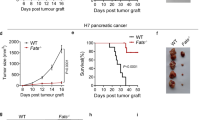
Abstract
The progression of colitis-associated cancer (CAC) is strongly associated with bone marrow-derived immunosuppressive cells (MDSCs). Although CAC could be suppressed by inducing MDSCs apoptosis, the immunosuppressive tumor microenvironment (TME) maintains immune homeostasis by upregulating M2-type tumor-associated macrophages (TAMs), thus leading to adaptive immune tolerance. Herein, we develop a dendritic cell (DC)-liposome conjugate to reverse immunosuppressive TME, showing remarkable efficiency against colorectal cancer. The DC-liposome conjugate is fabricated by conjugating resolvin E1-loaded liposomes with Fas ligand-transfected DCs, which eliminates tumor-infiltrated Fas+ MDSCs and enhances TAM phagocytosis in tumors. It shows significant therapeutic effects in preclinical CAC models and alleviates severe colitis when combined with immune checkpoint inhibitors. This study provides a feasible and customized cell-drug conjugate to overcome immunosuppressive TME for enhancing CAC immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information. The raw datasets generated during the study are available for research purposes from the corresponding authors on reasonable request. Source data are provided with this paper.
References
Nunes L, Li F, Wu M, Luo T, Hammarström K, Torell E, et al. Prognostic genome and transcriptome signatures in colorectal cancers. Nature. 2024;633:137–46.
Levi-Galibov O, Lavon H, Wassermann-Dozorets R, Pevsner-Fischer M, Mayer S, Wershof E, et al. Heat Shock Factor 1-dependent extracellular matrix remodeling mediates the transition from chronic intestinal inflammation to colon cancer. Nat Commun. 2020;11:6245.
Shah SC, Itzkowitz SH. Colorectal cancer in inflammatory bowel disease: mechanisms and management. Gastroenterology. 2022;162:715–30.
Robles AI, Traverso G, Zhang M, Roberts NJ, Khan MA, Joseph C, et al. Whole-exome sequencing analyses of inflammatory bowel disease-associated colorectal cancers. Gastroenterology. 2016;150:931–43.
Dan WY, Zhou GZ, Peng LH, Pan F. Update and latest advances in mechanisms and management of colitis-associated colorectal cancer. World J Gastrointest Oncol. 2023;15:1317–31.
Schmitt M, Greten FR. The inflammatory pathogenesis of colorectal cancer. Nat Rev Immunol. 2021;21:653–67.
Baker AM, Cross W, Curtius K, Al Bakir I, Choi CR, Davis HL, et al. Evolutionary history of human colitis-associated colorectal cancer. Gut. 2019;68:985–95.
Ocansey DKW, Qiu W, Wang J, Yan Y, Qian H, Zhang X, et al. The achievements and challenges of mesenchymal stem cell-based therapy in inflammatory bowel disease and its associated colorectal cancer. Stem Cells Int. 2020;2020:7819824.
Wang K, Wang Y, Yin K. Role played by MDSC in colitis-associated colorectal cancer and potential therapeutic strategies. J Cancer Res Clin Oncol. 2024;150:243.
Segal AW. Studies on patients establish Crohn’s disease as a manifestation of impaired innate immunity. J Intern Med. 2019;286:373–88.
Elliott LA, Doherty GA, Sheahan K, Ryan EJ. Human tumor-infiltrating myeloid cells: phenotypic and functional diversity. Front Immunol. 2017;8:86.
Chen Z, Zhang X, Xing Z, Lv S, Huang L, Liu J, et al. OLFM4 deficiency delays the progression of colitis to colorectal cancer by abrogating PMN-MDSCs recruitment. Oncogene. 2022;41:3131–50.
Lasser SA, Ozbay Kurt FG, Arkhypov I, Utikal J, Umansky V. Myeloid-derived suppressor cells in cancer and cancer therapy. Nat Rev Clin Oncol. 2024;21:147–64.
Mei Y, Zhu Y, Yong KSM, Hanafi ZB, Gong H, Liu Y, et al. IL-37 dampens immunosuppressive functions of MDSCs via metabolic reprogramming in the tumor microenvironment. Cell Rep. 2024;43:113835.
Li S, Xu F, Ren X, Tan L, Fu C, Wu Q, et al. H2S-reactivating antitumor immune response after microwave thermal therapy for long-term tumor suppression. ACS Nano. 2023;17:19242–53.
Xia Y, Rao L, Yao H, Wang Z, Ning P, Chen X. Engineering macrophages for cancer immunotherapy and drug delivery. Adv Mater. 2020;32:2002054.
Cheng S, Li Z, Gao R, Xing B, Gao Y, Yang Y, et al. A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell. 2021;184:792–809.
Zhang L, Li Z, Skrzypczynska KM, Fang Q, Zhang W, O’Brien SA, et al. Single-cell analyses inform mechanisms of myeloid-targeted therapies in colon cancer. Cell. 2020;181:442–59.
Xiao CX, Wang HH, Shi Y, Li P, Liu YP, Ren JL, et al. Distribution of bone-marrow-derived endothelial and immune cells in a murine colitis-associated colorectal cancer model. PLoS One. 2013;8:e73666.
Zundler S, Günther C, Kremer AE, Zaiss MM, Rothhammer V, Neurath MF. Gut immune cell trafficking: inter-organ communication and immune-mediated inflammation. Nat Rev Gastroenterol Hepatol. 2023;20:50–64.
Herová M, Schmid M, Gemperle C, Hersberger M. ChemR23, the receptor for chemerin and resolvin E1, is expressed and functional on M1 but not on M2 macrophages. J Immunol. 2015;194:2330–7.
Sinha P, Chornoguz O, Clements VK, Artemenko KA, Zubarev RA, Ostrand-Rosenberg S. Myeloid-derived suppressor cells express the death receptor Fas and apoptose in response to T cell-expressed FasL. Blood. 2011;117:5381–90.
Ohira T, Arita M, Omori K, Recchiuti A, Van Dyke TE, Serhan CN. Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J Biol Chem. 2010;285:3451–61.
De Matteis R, Flak MB, Gonzalez-Nunez M, Austin-Williams S, Palmas F, Colas RA, et al. Aspirin activates resolution pathways to reprogram T cell and macrophage responses in colitis-associated colorectal cancer. Sci Adv. 2022;8:abl5420.
Dooling LJ, Andrechak JC, Hayes BH, Kadu S, Zhang W, Pan R, et al. Cooperative phagocytosis of solid tumours by macrophages triggers durable anti-tumour responses. Nat Biomed Eng. 2023;7:1081–96.
Yan G, Zhao H, Zhang Q, Zhou Y, Wu L, Lei J, et al. A RIPK3-PGE2 circuit mediates myeloid-derived suppressor cell-potentiated colorectal carcinogenesis. Cancer Res. 2018;78:5586–99.
Hirano T, Hirayama D, Wagatsuma K, Yamakawa T, Yokoyama Y, Nakase H. Immunological mechanisms in inflammation-associated colon carcinogenesis. Int J Mol Sci. 2020;21:3062.
Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8:328rv4.
Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20:651–68.
Som A, Mandaliya R, Alsaadi D, Farshidpour M, Charabaty A, Malhotra N, et al. Immune checkpoint inhibitor-induced colitis: A comprehensive review. World J Clin Cases. 2019;7:405–18.
Velikova T, Krastev B, Gulinac M, Zashev M, Graklanov V, Peruhova M. New strategies in the diagnosis and treatment of immune-checkpoint inhibitor-mediated colitis. World J Clin Cases. 2024;12:1050–62.
Lo BC, Kryczek I, Yu J, Vatan L, Caruso R, Matsumoto M, et al. Microbiota-dependent activation of CD4+ T cells induces CTLA-4 blockade-associated colitis via Fcγ receptors. Science. 2024;383:62–70.
Hailemichael Y, Johnson DH, Abdel-Wahab N, Foo WC, Bentebibel SE, Daher M, et al. Interleukin-6 blockade abrogates immunotherapy toxicity and promotes tumor immunity. Cancer Cell. 2022;40:509–23.
Acknowledgements
We thank the National Center for Protein Science Shanghai for the platform of Fortessa flow cytometer, Leica TCS SP8 confocal microscope and 200 kV TEM. We are grateful to Y. Wang for FACS training, F. Liu for training in using confocal microscopy and J. Duan for operating 200 kV TEM (TF20, FEI). Financial supports from National Key R&D Program of China (2022YFC3401404), National Natural Science Foundation of China (32170935), Strategic Priority Research Program of the Chinese Academy of Grant (XDC0290302), Science and Technology Commission of Shanghai Municipality (24J22800600) and Shandong Laboratory Program (SYS202205) are gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
WZY and DGW conceived and designed the project, analyzed and interpreted data, and wrote and edited the manuscript; WZY carried out the characterization of the system in vitro and in vivo; WZY and DGW conducted proteomics and analyzed data; WZY, XDQ, XXX, RP, DY and ZWZ contributed to the animal experiment. DGW and YPL supervised the research; All authors discussed the results and reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yi, Wz., Qian, Xd., Xu, Xx. et al. Dendritic cell-liposome conjugates reverse immunosuppressive tumor microenvironment for inhibiting colitis-associated colorectal cancer. Acta Pharmacol Sin (2025). https://doi.org/10.1038/s41401-025-01614-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41401-025-01614-7