Abstract
Cholestatic liver disease is characterized by highly accumulated bile acids and cholangiocyte proliferation, resulting in the development of fibrosis, cirrhosis, and ultimately liver failure necessitating liver transplantation. Calcium (Ca2+) signaling is commonly dysregulated in cholestasis and serves as an important regulator mediating cell proliferation. However, the role of Ca2+-mediated cholangiocyte proliferation and treatment strategies in bile duct ligation (BDL)-induced liver injury remains poorly understood. By integrating transcriptomic analysis with molecular biology techniques, we explored the mechanisms of liver injury across BDL animal models, primary cholangiocytes, and human intrahepatic biliary epithelial cholangiocytes. Here, we found that a natural ingredient, senkyunolide A (SenA), effectively alleviated cholestasis-induced Ca2+ release from ER by inhibiting RYR channel, thereby preventing FIP200-mediated ER autophagy in response to Ca2+ transients on the cytosolic ER surface. Increased cytosolic Ca2+ further triggered ER stress, cholangiocyte cycle progression, and ductular reaction (DR). Importantly, SenA reversed the above process through its binding to chloride Channel CLIC Like 1 (CLCC1) for ubiquitination, thereby inhibiting CLCC1 activity and ER Ca2+ release. si-CLCC1-loaded liposomes targeting cholangiocytes enhanced the anti-DR effects of SenA. Collectively, by controlling ER release of Ca2+ in cholangiocytes, SenA presents potential for the development of therapeutic strategies aimed at addressing cholestatic fibrosis.

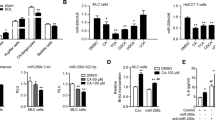
SenA inhibited Ca2+-mediated cholangiocyte proliferation by binding to and promoting the ubiquitination of CLCC1, thereby alleviating cholestatic liver fibrosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Chiang JYL, Ferrell JM. Bile acid biology, pathophysiology, and therapeutics. Clin Liver Dis. 2020;15:91–4.
Hasegawa S, Yoneda M, Kurita Y, Nogami A, Honda Y, Hosono K, et al. Cholestatic liver disease: current treatment strategies and new therapeutic agents. Drugs. 2021;81:1181–92.
Wu H, Chen C, Ziani S, Nelson LJ, Ávila MA, Nevzorova YA, et al. Fibrotic events in the progression of cholestatic liver disease. Cells. 2021;10:1107.
Zeng J, Fan J, Zhou H. Bile acid-mediated signaling in cholestatic liver diseases. Cell Biosci. 2023;13:77.
Xu YN, Xu W, Zhang X, Wang DY, Zheng XR, Liu W, et al. BM-MSCs overexpressing the Numb enhance the therapeutic effect on cholestatic liver fibrosis by inhibiting the ductular reaction. Stem Cell Res Ther. 2023;14:45.
Mavila N, Siraganahalli Eshwaraiah M, Kennedy J. Ductular reactions in liver injury, regeneration, and disease progression—an overview. Cells. 2024;13:579.
Wang M, Zhang M, Fu L, Lin J, Zhou X, Zhou P, et al. Liver-targeted delivery of TSG-6 by calcium phosphate nanoparticles for the management of liver fibrosis. Theranostics. 2020;10:36–49.
Xiao WC, Zhang J, Chen SL, Shi YJ, Xiao F, An W. Alleviation of palmitic acid-induced endoplasmic reticulum stress by augmenter of liver regeneration through IP3R-controlled Ca2+ release. J Cell Physiol. 2018;233:6148–57.
López-Neblina F, Toledo-Pereyra LH, Toledo AH, Walsh J. Ryanodine receptor antagonism protects the ischemic liver and modulates TNF-alpha and IL-10. J Surg Res. 2007;140:121–8.
Corazzari M, Gagliardi M, Fimia GM, Piacentini M. Endoplasmic reticulum stress, unfolded protein response, and cancer cell fate. Front Oncol. 2017;7:78.
Trampert DC, Nathanson MH. Regulation of bile secretion by calcium signaling in health and disease. Biochim Biophys Acta Mol Cell Res. 2018;1865:1761–70.
Cai X, Yu X, Yang J, Lu L, Hua N, Duan X, et al. TRPM2 regulates cell cycle through the Ca2+-CaM-CaMKII signaling pathway to promote HCC. Hepatol Commun. 2023;7:e0101.
Patergnani S, Danese A, Bouhamida E, Aguiari G, Previati M, Pinton P, et al. Various aspects of calcium signaling in the regulation of apoptosis, autophagy, cell proliferation, and cancer. Int J Mol Sci. 2020;21:8323.
Kondratskyi A, Kondratska K, Skryma R, Klionsky DJ, Prevarskaya N. Ion channels in the regulation of autophagy. Autophagy. 2018;14:3–21.
Zheng Q, Chen Y, Chen D, Zhao H, Feng Y, Meng Q, et al. Calcium transients on the ER surface trigger liquid-liquid phase separation of FIP200 to specify autophagosome initiation sites. Cell. 2022;185:4082–98.e22.
Petrescu AD, Grant S, Williams E, Frampton G, Reinhart EH, Nguyen A, et al. Ghrelin reverses ductular reaction and hepatic fibrosis in a rodent model of cholestasis. Sci Rep. 2020;10:16024.
Lu L, Lu T, Wu Y, Wang Y, Ke X, Yang R. Research on the effectiveness and material basis of Ligusticum chuanxiong in alleviating acute liver injury. J Ethnopharmacol. 2023;314:116643.
Li Y, Ma Z, Ding M, Jia K, Xu B, Zhou F, et al. Chuanxiong Rhizoma extracts prevent cholestatic liver injury by targeting H3K9ac-mediated and cholangiocyte-derived secretory protein PAI-1 and FN. Chin J Nat Med. 2023;21:694–709.
Wu JZ, Li YJ, Huang GR, Xu B, Zhou F, Liu RP, et al. Mechanisms exploration of Angelicae Sinensis Radix and Ligusticum Chuanxiong Rhizoma herb-pair for liver fibrosis prevention based on network pharmacology and experimental pharmacology. Chin J Nat Med. 2021;19:241–54.
Li Y, Li F, Ding M, Ma Z, Li S, Qu J, et al. Chuanxiong Rhizoma extracts prevent liver fibrosis via targeting CTCF-c-MYC-H19 pathway. Chin Herb Med. 2024;16:82–93.
Huang Y, Ni N, Hong Y, Lin X, Feng Y, Shen L. Progress in traditional Chinese medicine for the treatment of migraine. Am J Chin Med. 2020;48:1731–48.
Shao M, Lv D, Zhou K, Sun H, Wang Z. Senkyunolide A inhibits the progression of osteoarthritis by inhibiting the NLRP3 signalling pathway. Pharm Biol. 2022;60:535–42.
Lei W, Deng YF, Hu XY, Ni JN, Jiang M, Bai G. Phthalides, senkyunolide A and ligustilide, show immunomodulatory effect in improving atherosclerosis, through inhibiting AP-1 and NF-κB expression. Biomed Pharmacother. 2019;117:109074.
Duan S, Li X, Han J, Yang Y, Luo R, Cai Y, et al. Transcriptotype-driven discovery of apigenin as a therapy against cholestatic liver fibrosis: through inhibition of PANoptosis and following type-I interferon responses. Antioxidants. 2024;13:256.
Li X, Liu R, Yang J, Sun L, Zhang L, Jiang Z, et al. The role of long noncoding RNA H19 in gender disparity of cholestatic liver injury in multidrug resistance 2 gene knockout mice. Hepatology. 2017;66:869–84.
Ji R, Chen J, Xie Y, Dou X, Qing B, Liu Z, et al. Multi-omics profiling of cholangiocytes reveals sex-specific chromatin state dynamics during hepatic cystogenesis in polycystic liver disease. J Hepatol. 2023;78:754–69.
Jia K, Zhang Y, Luo R, Liu R, Li Y, Wu J, et al. Acteoside ameliorates hepatic ischemia-reperfusion injury via reversing the senescent fate of liver sinusoidal endothelial cells and restoring compromised sinusoidal networks. Int J Biol Sci. 2023;19:4967–88.
Hu Y, Bao X, Zhang Z, Chen L, Liang Y, Qu Y, et al. Hepatic progenitor cell-originated ductular reaction facilitates liver fibrosis through activation of hedgehog signaling. Theranostics. 2024;14:2379–95.
Alpini G, Glaser SS, Ueno Y, Rodgers R, Phinizy JL, Francis H, et al. Bile acid feeding induces cholangiocyte proliferation and secretion: evidence for bile acid-regulated ductal secretion. Gastroenterology. 1999;116:179–86.
Wang Y, Aoki H, Yang J, Peng K, Liu R, Li X, et al. The role of sphingosine 1-phosphate receptor 2 in bile-acid-induced cholangiocyte proliferation and cholestasis-induced liver injury in mice. Hepatology. 2017;65:2005–18.
Santos-Laso A, Izquierdo-Sanchez L, Rodrigues PM, Huang BQ, Azkargorta M, Lapitz A, et al. Proteostasis disturbances and endoplasmic reticulum stress contribute to polycystic liver disease: new therapeutic targets. Liver Int. 2020;40:1670–85.
Che Y, Xu W, Ding C, He T, Xu X, Shuai Y, et al. Bile acids target mitofusin 2 to differentially regulate innate immunity in physiological versus cholestatic conditions. Cell Rep. 2023;42:112011.
Hu M, Feng X, Liu Q, Liu S, Huang F, Xu H. The ion channels of endomembranes. Physiol Rev. 2024;104:1335–85.
Filadi R, Greotti E, Pizzo P. Highlighting the endoplasmic reticulum-mitochondria connection: focus on Mitofusin 2. Pharmacol Res. 2018;128:42–51.
Park JH, Kim HK, Jung H, Kim KH, Kang MS, Hong JH, et al. NecroX-5 prevents breast cancer metastasis by AKT inhibition via reducing intracellular calcium levels. Int J Oncol. 2017;50:185–92.
Guo L, Mao Q, He J, Liu X, Piao X, Luo L, et al. Disruption of ER ion homeostasis maintained by an ER anion channel CLCC1 contributes to ALS-like pathologies. Cell Res. 2023;33:497–515.
Banales JM, Huebert RC, Karlsen T, Strazzabosco M, LaRusso NF, Gores GJ. Cholangiocyte pathobiology. Nat Rev Gastroenterol Hepatol. 2019;16:269–81.
Arruda AP, Hotamisligil GS. Calcium homeostasis and organelle function in the pathogenesis of obesity and diabetes. Cell Metab. 2015;22:381–97.
Luo S, Zhao X, Jiang J, Deng B, Liu S, Xu H, et al. Piezo1 specific deletion in macrophage protects the progression of liver fibrosis in mice. Theranostics. 2023;13:5418–34.
Li W, Chen JY, Sun C, Sparks RP, Pantano L, Rahman RU, et al. Nanchangmycin regulates FYN, PTK2, and MAPK1/3 to control the fibrotic activity of human hepatic stellate cells. eLife. 2022;11:e74513.
Ali ES, Rychkov GY, Barritt GJ. Deranged hepatocyte intracellular Ca2+ homeostasis and the progression of non-alcoholic fatty liver disease to hepatocellular carcinoma. Cell Calcium. 2019;82:102057.
Guo M, Liu R, Zhang F, Qu J, Yang Y, Li X. A new perspective on liver diseases: Focusing on the mitochondria-associated endoplasmic reticulum membranes. Pharmacol Res. 2024;208:107409.
Raut SK, Singh K, Sanghvi S, Loyo-Celis V, Varghese L, Singh ER, et al. Chloride ions in health and disease. Biosci Rep. 2024;44:BSR20240029.
Gao J, Wei B, de Assuncao TM, Liu Z, Hu X, Ibrahim S, et al. Hepatic stellate cell autophagy inhibits extracellular vesicle release to attenuate liver fibrosis. J Hepatol. 2020;73:1144–54.
Li Q, Sheng J, Baruscotti M, Liu Z, Wang Y, Zhao L. Identification of Senkyunolide I as a novel modulator of hepatic steatosis and PPARα signaling in zebrafish and hamster models. J Ethnopharmacol. 2025;336:118743.
Acknowledgements
This work was supported by grants from the Scientific Research Innovation Capability Support Project for Young Faculty (ZYGXQNJSKYCXNLZCXM-H4); National Natural Science Foundation of China (Grant No. 82322075 to RPL and 82274186 to XJYL); National High-Level Talents Special Support Program to XJYL; Beijing Municipal Science & Technology Commission (Grant No. 7212174 to XJYL); National Key Research and Development Program on Modernization of Traditional Chinese Medicine to XJYL; Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (Grant No. ZYYCXTD-C-202006 to XJYL).
Author information
Authors and Affiliations
Contributions
YJL: methodology, formal analysis, writing—original draft. MYG: methodology, writing—original draft. WQQ: validation. JNL: methodology. YFL: validation. FKZ: methodology. XYX: methodology. RPL: investigation. SL: methodology. JRQ: investigation. LW: methodology. XJYL: conceptualization, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Yj., Guo, My., Qin, Wq. et al. Senkyunolide A ameliorates cholestatic liver fibrosis by controlling CLCC1-mediated endoplasmic reticulum Ca2+ release. Acta Pharmacol Sin 46, 3257–3272 (2025). https://doi.org/10.1038/s41401-025-01615-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-025-01615-6
Keywords
This article is cited by
-
Mitochondrial Calcium Channels and MAM Interaction in Calcium Homeostasis Dysregulation in Parkinson’s Disease
Neurochemical Research (2025)