Abstract
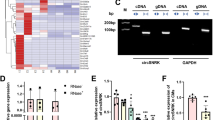
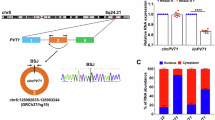
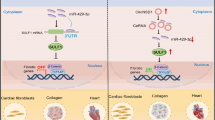
Increasing evidence shows that microRNAs (miRNAs) are functionally associated with cardiac remodeling. Functionally, some cytoplasmic circRNAs may act as miRNA sponges to inhibit functions of the combined miRNAs. Our previous study showed that miR-199a-5p and -3p promote cardiac hypertrophy and fibrosis. In this study, we designed and synthesized a novel circularized RNA sponge, circSP199a, and evaluated the therapeutic effects of circSP199a against cardiac hypertrophy and fibrosis. The synthesized circSP199a included 6 repeats of reverse complements of seed sequences of miR-199a-5p and -3p with 515 nt in length. We showed that the synthesized circSP199a and expression vector-mediated circSP199a expression inhibited cardiomyocyte hypertrophy and mitigated the fibrotic phenotypes in neonatal mouse cardiac fibroblasts and human cardiac organoid fibrosis via the combination of miR-199a-5p and -3p. Furthermore, intravenous injection of AAV9-circSP199a for 21 days in advance significantly ameliorated transverse aortic constriction-induced cardiac injury and remodeling in mice. We demonstrated that circSP199a blocked the functions of miR-199a-5p and -3p to enhance the expression of target genes of PGC-1α, Rb1, Sirt1 and Smad1 both in vitro and in vivo. These results provide new insights into the development of RNA sponge-based therapies for cardiac hypertrophy and fibrosis.

The artificial circSP199a functions as a novel inhibitor of miR-199a-5p and -3p, exogenous provision of circSP199a specifically sponges miR-199a-5p and -3p to block their functions with subsequent upregulations of the target genes, including PGC-1α, Rb1, SIRT1 and Smad1, to mitigate the pathological cardiac hypertrophy and fibrosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Bertero E, Maack C. Metabolic remodeling in heart failure. Nat Rev Cardiol. 2018;15:457–70.
Perrino C, Naga Prasad SV, Mao L, Noma T, Yan Z, Kim HS, et al. Intermittent pressure overload triggers hypertrophy-independent cardiac dysfunction and vascular rarefaction. J Clin Invest. 2006;116:1547–60.
Zhou SS, Jin JP, Wang JQ, Zhang ZG, Freedman JH, Zheng Y, et al. miRNAs in cardiovascular diseases: potential biomarkers, therapeutic targets and challenges. Acta Pharmacol Sin. 2018;39:1073–84.
Dzau VJ, Hodgkinson CP. RNA therapeutics for the cardiovascular system. Circulation. 2024;149:707–16.
Xiao M, Li J, Li W, Wang Y, Wu F, Xi Y, et al. MicroRNAs activate gene transcription epigenetically as an enhancer trigger. RNA Biol. 2017;14:1326–34.
Liang Y, Zou Q, Yu W. Steering against wind: a new network of namiRNAs and enhancers. Genomics Proteom Bioinforma. 2017;15:331–7.
Li J, Salvador AM, Li G, Valkov N, Ziegler O, Yeri A, et al. Mir-30d regulates cardiac remodeling by intracellular and paracrine signaling. Circ Res. 2021;128:e1–e23.
Gao L, Qiu F, Cao H, Li H, Dai G, Ma T, et al. Therapeutic delivery of microRNA-125a-5p oligonucleotides improves recovery from myocardial ischemia/reperfusion injury in mice and swine. Theranostics. 2023;13:685–703.
Ramanujam D, Schön AP, Beck C, Vaccarello P, Felician G, Dueck A, et al. MicroRNA-21-dependent macrophage-to-fibroblast signaling determines the cardiac response to pressure overload. Circulation. 2021;143:1513–25.
Li G, Shao Y, Guo HC, Zhi Y, Qiao B, Ma K, et al. MicroRNA-27b-3p down-regulates FGF1 and aggravates pathological cardiac remodelling. Cardiovasc Res. 2022;118:2139–51.
Liu X, Li H, Hastings MH, Xiao C, Damilano F, Platt C, et al. miR-222 inhibits pathological cardiac hypertrophy and heart failure. Cardiovasc Res. 2024;120:262–72.
Zeng N, Huang YQ, Yan YM, Hu ZQ, Zhang Z, Feng JX, et al. Diverging targets mediate the pathological role of miR-199a-5p and miR-199a-3p by promoting cardiac hypertrophy and fibrosis. Mol Ther Nucleic Acids. 2021;26:1035–50.
Jeck WR, Sharpless NE. Detecting and characterizing circular RNAs. Nat Biotechnol. 2014;32:453–61.
Zhang C, Huo ST, Wu Z, Chen L, Wen C, Chen H, et al. Rapid development of targeting circRNAs in cardiovascular diseases. Mol Ther Nucleic Acids. 2020;21:568–76.
Liu X, Abraham JM, Cheng Y, Wang Z, Wang Z, Zhang G, et al. Synthetic circular RNA functions as a miR-21 sponge to suppress gastric carcinoma cell proliferation. Mol Ther Nucleic Acids. 2018;13:312–21.
Wang Z, Ma K, Cheng Y, Abraham JM, Liu X, Ke X, et al. Synthetic circular multi-miR sponge simultaneously inhibits miR-21 and miR-93 in esophageal carcinoma. Lab Invest. 2019;99:1442–53.
Jost I, Shalamova LA, Gerresheim GK, Niepmann M, Bindereif A, Rossbach O. Functional sequestration of microRNA-122 from Hepatitis C Virus by circular RNA sponges. RNA Biol. 2018;15:1032–9.
Lavenniah A, Luu TDA, Li YP, Lim TB, Jiang J, Ackers-Johnson M, et al. Engineered circular RNA sponges act as miRNA inhibitors to attenuate pressure overload-induced cardiac hypertrophy. Mol Ther. 2020;28:1506–17.
Tang CM, Liu FZ, Zhu JN, Fu YH, Lin QX, Deng CY, et al. Myocyte-specific enhancer factor 2C: a novel target gene of miR-214-3p in suppressing angiotensin II-induced cardiomyocyte hypertrophy. Sci Rep. 2016;6:36146.
Liang JN, Zou X, Fang XH, Xu JD, Xiao Z, Zhu JN, et al. The Smad3-miR-29b/miR-29c axis mediates the protective effect of macrophage migration inhibitory factor against cardiac fibrosis. Biochim Biophys Acta Mol Basis Dis. 2019;1865:2441–50.
Li H, Xu JD, Fang XH, Zhu JN, Yang J, Pan R, et al. Circular RNA circRNA_000203 aggravates cardiac hypertrophy via suppressing miR-26b-5p and miR-140-3p binding to Gata4. Cardiovasc Res. 2020;116:1323–34.
Lewis-Israeli YR, Wasserman AH, Gabalski MA, Volmert BD, Ming Y, Ball KA, et al. Self-assembling human heart organoids for the modeling of cardiac development and congenital heart disease. Nat Commun. 2021;12:5142.
Yang J, Lei W, Xiao Y, Tan S, Yang J, Lin Y, et al. Generation of human vascularized and chambered cardiac organoids for cardiac disease modelling and drug evaluation. Cell Prolif. 2024;57:e13631.
Huang S, Zou X, Zhu JN, Fu YH, Lin QX, Liang YY, et al. Attenuation of microRNA-16 derepresses the cyclins D1, D2 and E1 to provoke cardiomyocyte hypertrophy. J Cell Mol Med. 2015;19:608–19.
Huang C, Shan G. What happens at or after transcription: Insights into circRNA biogenesis and function. Transcription. 2015;6:61–64.
Ye Z, Jin H, Qian Q. Argonaute 2: a novel rising star in cancer research. J Cancer. 2015;6:877–82.
Segers VFM, Brutsaert DL, De Keulenaer GW. Cardiac remodeling: endothelial cells have more to say than just NO. Front Physiol. 2018;9:382.
Talman V, Kivelä R. Cardiomyocyte-endothelial cell interactions in cardiac remodeling and regeneration. Front Cardiovasc Med. 2018;5:101.
Vishnoi A, Rani S. miRNA biogenesis and regulation of diseases: an updated overview. Methods Mol Biol. 2023;2595:1–12.
Couzin J. MicroRNAs make big impression in disease after disease. Science. 2008;319:1782–4.
Diener C, Keller A, Meese E. Emerging concepts of miRNA therapeutics: from cells to clinic. Trends Genet. 2022;38:613–26.
Ho PTB, Clark IM, Le LTT. MicroRNA-based diagnosis and therapy. Int J Mol Sci. 2022;23:7167.
Seyhan AA. Trials and tribulations of microRNA therapeutics. Int J Mol Sci. 2024;25:1469.
Ebert MS, Sharp PA. MicroRNA sponges: progress and possibilities. RNA. 2010;16:2043–50.
Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods. 2007;4:721–6.
Li D, Zhang J, Li J. Role of miRNA sponges in hepatocellular carcinoma. Clin Chim Acta. 2020;500:10–19.
Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015;4:e05005.
Lin KH, Kumar VB, Shanmugam T, Shibu MA, Chen RJ, Kuo CH, et al. miR-145-5p targets paxillin to attenuate angiotensin II-induced pathological cardiac hypertrophy via downregulation of Rac1, pJNK, p-c-Jun, NFATc3, ANP and by Sirt-1 upregulation. Mol Cell Biochem. 2021;476:3253–60.
Zhou W, Ji L, Liu X, Tu D, Shi N, Yangqu W, et al. AIFM1, negatively regulated by miR-145-5p, aggravates hypoxia-induced cardiomyocyte injury. Biomed J. 2022;45:870–82.
Zhou Q, Deng J, Yao J, Song J, Meng D, Zhu Y, et al. Exercise downregulates HIPK2 and HIPK2 inhibition protects against myocardial infarction. EBioMedicine. 2021;74:103713.
Riehle C, Abel ED. PGC-1 proteins and heart failure. Trends Cardiovasc Med. 2012;22:98–105.
Wang Y, Zhao R, Wu C, Liang X, He L, Wang L, et al. Activation of the sirtuin silent information regulator 1 pathway inhibits pathological myocardial remodeling. Front Pharmacol. 2023;14:1111320.
Humeres C, Venugopal H, Frangogiannis NG. Smad-dependent pathways in the infarcted and failing heart. Curr Opin Pharmacol. 2022;64:102207.
Zhu L, Liu K, Feng Q, Liao Y. Cardiac organoids: a 3D technology for modeling heart development and disease. Stem Cell Rev Rep. 2022;18:2593–605.
Cho J, Lee H, Rah W, Chang HJ, Yoon YS. From engineered heart tissue to cardiac organoid. Theranostics. 2022;12:2758–72.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (No. 82200325, 82070254, 82300277, 82400331 and 82470253), High-level Hospital Construction Project of Guangdong Provincial People’s Hospital (No. DFJH201902, No. DFJHBF202102), Basic and Applied Basic Research Foundation of Guangdong Province (No. 2023A1515010201, 2025A1515011249, 2025A1515010873). We appreciate BioRender (https://www.biorender.com) for help in figure production during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
XHF and ZXS conceived the project. HYW, CMZ, YG, HLZ, YTH and MZZ performed laboratory experiments. YHW and WYD performed the viral preparation and infection. XYL and LZ analyzed bioinformatics data. HLZ, HL, YL and JNZ prepared the figures. JDX, NM and YPL made the statistical analysis. XLZ, XHF and ZXS supervised the research. HYW and ZXS wrote and revised the manuscript. All authors have read and approved the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, Hy., Zhou, Cm., Gao, Y. et al. circSP199a, a circularized RNA sponge targeting miR-199a-5p and -3p, mitigates mouse cardiac hypertrophy and fibrosis. Acta Pharmacol Sin 47, 86–102 (2026). https://doi.org/10.1038/s41401-025-01620-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-025-01620-9