Abstract
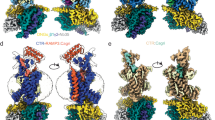
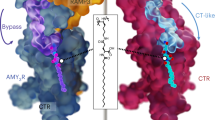
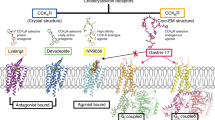
The global obesity epidemic and its associated metabolic disorders urgently require more effective therapeutic interventions, particularly multi-pathway targeting therapies. Cagrilintide (Cagri), functioning as a dual amylin receptor (AMYRs) and calcitonin receptor (CTR) agonist (DACRA), demonstrates significant efficacy in obesity treatment, although its structural activation mechanism remains unclear. This study elucidates the non-selective activation mechanism by determining cryo-EM structures of Cagri bound to AMY1R-Gs and CTR-Gs complexes. Cagri adopts similar “bypass” binding modes in both receptors, which is distinct from other existing DACRAs that primarily achieve extended half-life through N-terminal lipid modification. Key molecular features include the F23Cagri residue anchoring the peptide at the receptor transmembrane (TM) bundle level and the micelle, an E14-R17 intramolecular salt bridge enhancing helical stability, and C-terminal P37Cagri interaction with the receptor ECD. These features collectively enable non-specific binding and activation across different receptors. Both structural and functional analyses revealed Cagri’s non-selective activation of Gs signaling pathways through CTR and AMY1R. These findings provide a comprehensive structural framework for developing next-generation anti-obesity drugs based on dual receptor activation mechanisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The atomic coordinates for the Cagri‒CTR‒Gs and Cagri‒AMY1R‒Gs complexes have been deposited in the Protein Data Bank (PDB) under accession codes 9UWM and 9UWQ, respectively. Cryo-EM maps have been deposited in the Electron Microscopy Data Bank under the following accession codes: for the Cagri‒CTR‒Gs complex, EMD-64557 (raw map), EMD-64558 (receptor-focused refinement map), and EMD-64560 (composite map); for the Cagri‒AMY1R‒Gs complex, EMD-64562 (raw map), EMD-64561 (receptor-focused refinement map), and EMD-64563 (composite map).
References
Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15:288–98.
Artasensi A, Pedretti A, Vistoli G, Fumagalli L. Type 2 diabetes mellitus: a review of multi-target drugs. Molecules. 2020;25:1987.
Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038–48.
Zhao F, Zhou Q, Cong Z, Hang K, Zou X, Zhang C, et al. Structural insights into multiplexed pharmacological actions of tirzepatide and peptide 20 at the GIP, GLP-1 or glucagon receptors. Nat Commun. 2022;13:1057.
Eržen S, Tonin G, Jurišić Eržen D, Klen J. Amylin, another important neuroendocrine hormone for the treatment of diabesity. Int J Mol Sci 2024;25:1517.
Hartter E, Svoboda T, Ludvik B, Schuller M, Lell B, Kuenburg E, et al. Basal and stimulated plasma levels of pancreatic amylin indicate its co-secretion with insulin in humans. Diabetologia. 1991;34:52–4.
Butler PC, Chou J, Carter WB, Wang YN, Bu BH, Chang D, et al. Effects of meal ingestion on plasma amylin concentration in NIDDM and nondiabetic humans. Diabetes. 1990;39:752–6.
Pioszak AA, Hay DL. RAMPs as allosteric modulators of the calcitonin and calcitonin-like class B G protein-coupled receptors. Adv Pharmacol. 2020;88:115–41.
Kuwasako K, Hay DL, Nagata S, Murakami M, Kitamura K, Kato J. Functions of third extracellular loop and helix 8 of Family B GPCRs complexed with RAMPs and characteristics of their receptor trafficking. Curr Protein Pept Sci. 2013;14:416–28.
Lee SM, Hay DL, Pioszak AA. Calcitonin and amylin receptor peptide interaction mechanisms: insights into peptide-binding modes and allosteric modulation of the calcitonin receptor by receptor activity-modifying proteins. J Biol Chem. 2016;291:8686–700.
Mathiesen DS, Lund A, Vilsbøll T, Knop FK, Bagger JI. Amylin and calcitonin: potential therapeutic strategies to reduce body weight and liver fat. Front Endocrinol (Lausanne). 2020;11:617400.
Ryan G, Briscoe TA, Jobe L. Review of pramlintide as adjunctive therapy in treatment of type 1 and type 2 diabetes. Drug Des Devel Ther. 2009;2:203–14.
Ryan GJ, Jobe LJ, Martin R. Pramlintide in the treatment of type 1 and type 2 diabetes mellitus. Clin Ther. 2005;27:1500–12.
Knudsen LB, Lau J. The discovery and development of liraglutide and semaglutide. Front Endocrinol (Lausanne). 2019;10:155.
Lau DCW, Erichsen L, Francisco AM, Satylganova A, le Roux CW, McGowan B, et al. Once-weekly cagrilintide for weight management in people with overweight and obesity: a multicentre, randomised, double-blind, placebo-controlled and active-controlled, dose-finding phase 2 trial. Lancet. 2021;398:2160–72.
D’Ascanio AM, Mullally JA, Frishman WH. Cagrilintide: a long-acting amylin analog for the treatment of obesity. Cardiol Rev. 2024;32:83–90.
Enebo LB, Berthelsen KK, Kankam M, Lund MT, Rubino DM, Satylganova A, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of concomitant administration of multiple doses of cagrilintide with semaglutide 2·4 mg for weight management: a randomised, controlled, phase 1b trial. Lancet. 2021;397:1736–48.
Kruse T, Hansen JL, Dahl K, Schaffer L, Sensfuss U, Poulsen C, et al. Development of cagrilintide, a long-acting amylin analogue. J Med Chem. 2021;64:11183–94.
Frias JP, Deenadayalan S, Erichsen L, Knop FK, Lingvay I, Macura S, et al. Efficacy and safety of co-administered once-weekly cagrilintide 2·4 mg with once-weekly semaglutide 2·4 mg in type 2 diabetes: a multicentre, randomised, double-blind, active-controlled, phase 2 trial. Lancet. 2023;402:720–30.
Singh G, Krauthamer M, Bjalme-Evans M. Wegovy (semaglutide): a new weight loss drug for chronic weight management. J Investig Med. 2022;70:5–13.
Cao J, Belousoff MJ, Liang YL, Johnson RM, Josephs TM, Fletcher MM, et al. A structural basis for amylin receptor phenotype. Science. 2022;375:eabm9609.
Cao J, Belousoff MJ, Gerrard E, Danev R, Fletcher MM, Dal Maso E, et al. Structural insight into selectivity of amylin and calcitonin receptor agonists. Nat Chem Biol. 2024;20:162–9.
Dal Maso E, Glukhova A, Zhu Y, Garcia-Nafria J, Tate CG, Atanasio S, et al. The molecular control of calcitonin receptor signaling. ACS Pharmacol Transl Sci. 2019;2:31–51.
Liang YL, Khoshouei M, Radjainia M, Zhang Y, Glukhova A, Tarrasch J, et al. Phase-plate cryo-EM structure of a class B GPCR-G-protein complex. Nature. 2017;546:118–23.
Zhao LH, Ma S, Sutkeviciute I, Shen DD, Zhou XE, de Waal PW, et al. Structure and dynamics of the active human parathyroid hormone receptor-1. Science. 2019;364:148–53.
Nehmé R, Carpenter B, Singhal A, Strege A, Edwards PC, White CF, et al. Mini-G proteins: Novel tools for studying GPCRs in their active conformation. PLoS One. 2017;12:e0175642.
Zhao LH, Yuan QN, Dai AT, He XH, Chen CW, Zhang C, et al. Molecular recognition of two endogenous hormones by the human parathyroid hormone receptor-1. Acta Pharmacol Sin. 2023;44:1227–37.
Zhao LH, He Q, Yuan Q, Gu Y, He X, Shan H, et al. Conserved class B GPCR activation by a biased intracellular agonist. Nature. 2023;621:635–41.
Zheng SQ, Palovcak E, Armache JP, Verba KA, Cheng Y, Agard DA. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat Methods. 2017;14:331–2.
Rohou A, Grigorieff N. CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J Struct Biol. 2015;192:216–21.
Zivanov J, Nakane T, Scheres SHW. Estimation of high-order aberrations and anisotropic magnification from cryo-EM data sets in RELION-3.1. IUCrJ. 2020;7:253–67.
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, et al. UCSF Chimera—a visualization system for exploratory research and analysis. J Computat Chem. 2004;25:1605–12.
Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–32.
Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–21.
Wang JJ, Jin S, Zhang H, Xu Y, Hu W, Jiang Y, et al. Molecular recognition and activation of the prostacyclin receptor by anti-pulmonary arterial hypertension drugs. Sci Adv. 2024;10:eadk5184.
Zhao LH, Lin J, Ji SY, Zhou XE, Mao C, Shen DD, et al. Structure insights into selective coupling of G protein subtypes by a class B G protein-coupled receptor. Nat Commun. 2022;13:6670.
Hollenstein K, de Graaf C, Bortolato A, Wang MW, Marshall FH, Stevens RC. Insights into the structure of class B GPCRs. Trends Pharmacol Sci. 2014;35:12–22.
Cao J, Belousoff MJ, Johnson RM, Keov P, Mariam Z, Deganutti G, et al. Structural and dynamic features of cagrilintide binding to calcitonin and amylin receptors. Nat Commun. 2025;16:3389.
Dong M, Deganutti G, Piper SJ, Liang YL, Khoshouei M, Belousoff MJ, et al. Structure and dynamics of the active Gs-coupled human secretin receptor. Nat Commun. 2020;11:4137.
Ma S, Shen Q, Zhao LH, Mao C, Zhou XE, Shen DD, et al. Molecular basis for hormone recognition and activation of corticotropin-releasing factor receptors. Mol Cell. 2020;77:669–80.e4.
Josephs TM, Belousoff MJ, Liang YL, Piper SJ, Cao J, Garama DJ, et al. Structure and dynamics of the CGRP receptor in apo and peptide-bound forms. Science. 2021;372:eabf7258.
Acknowledgements
We acknowledge the use of cryo-EM facilities at the Advanced Center for Electron Microscopy, Shanghai Institute of Materia Medica, Chinese Academy of Sciences. We are grateful to WH and KW for technical assistance with data collection. This work was supported by the National Natural Science Foundation of China (32371255 and 32071203 to LHZ, 32130022 and 82121005 to HEX, 82404881 to QNY); the Natural Science Foundation of Shanghai (23ZR1475200 to LHZ); the National Key R&D Program of China (2022YFC2703105 to HEX, 2019YFA0904200); the CAS Strategic Priority Research Program (XDB37030103 to HEX); the Shanghai Municipal Science and Technology Major Project (2019SHZDZX02 to HEX); the Young Innovator Association of CAS (Y2022078 to LHZ); the Lingang Laboratory (LG-GG-202204-01 to HEX); the State Key Laboratory of Drug Research (SKLDR-2023-TT-04 to HEX).
Author information
Authors and Affiliations
Contributions
YMG designed the expression constructs and purified the protein complex under supervision of LHZ and HEX; LHZ and QNY prepared the grids. QNY performed cryo-EM data processing and model building. YMG constructed all the mutated plasmids and conducted functional studies under supervision of LHZ; XL carried out functional experiments supervised by LHZ; YMG, QH, and LHZ analyzed the structures. YMG prepared the figures and contributed to manuscript writing. All authors discussed and commented on the manuscript. LHZ and HEX wrote, revised, and finalized the manuscript, and jointly conceived, designed, and supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gu, Ym., Yuan, Qn., Li, X. et al. Structural and mechanistic insights into dual activation of cagrilintide in amylin and calcitonin receptors. Acta Pharmacol Sin 47, 162–172 (2026). https://doi.org/10.1038/s41401-025-01635-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-025-01635-2