Abstract
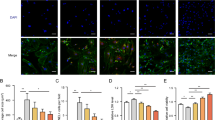
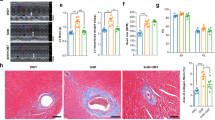
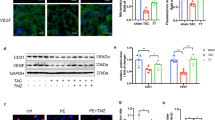
Pathological cardiac hypertrophy as a major contributor to heart failure is characterized by complicated mechanisms. Fumarate hydratase (FH) is a crucial enzyme in the tricarboxylic acid cycle. FH mutations and dysfunction have been implicated in various pathological processes including hereditary leiomyomatosis and renal cell cancer, neurodegenerative diseases, metabolic syndrome and cardiovascular diseases. In this study we investigated the role of FH in cardiac hypertrophy. Cardiac hypertrophy was induced in mice by transverse aortic constriction (TAC) surgery as well as in neonatal rat cardiomyocytes (NRCMs) by phenylephrine (PE) stimulation. We showed that the expression levels of FH were gradually increased with development of cardiac hypertrophy in TAC mice. Cardiomyocyte-specific overexpression of FH by intravenous injection of recombinant adeno-associated virus serotype 9 (AAV9) carrying FH two weeks before TAC surgery prevented the morphological changes, cardiac dysfunction and remodeling in TAC mice; FH overexpression also significantly attenuated PE-induced hypertrophy in NRCMs along with suppressed expression of hypertrophic markers ANP, BNP and β-MHC. We demonstrated that FH overexpression alleviated TAC-induced mitochondrial structural damage in cardiomyocytes and facilitated metabolic remodeling. RNA sequencing and untargeted metabolomics revealed that FH overexpression mitigated myocardial remodeling and mitochondrial metabolism dysfunction in TAC mice mainly by suppressing the transcription factor SREBP and reducing the gene expression of elongation of very long chain fatty acids protein 7 (Elovl7). Overexpression of Elovl7 reversed the protective effects of FH in both TAC mice and PE-stimulated NRCMs. Knockdown of the transcription factor SREBP reduced Elovl7 expression, thereby exerting cardioprotective effects. In conclusion, we demonstrate that FH overexpression prevents cardiac hypertrophy in mice by regulating glucose and lipid metabolism through the malate-SREBP-Elovl7 pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Oka T, Akazawa H, Naito AT, Komuro I. Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ Res. 2014;114:565–71. https://doi.org/10.1161/CIRCRESAHA.114.300507.
Ju J, Wang K, Liu F, Liu CY, Wang YH, Wang SC, et al. Crotonylation of NAE1 modulates cardiac hypertrophy via gelsolin neddylation. Circ Res. 2024;135:806–21. https://doi.org/10.1161/CIRCRESAHA.124.324733.
Nomura S, Satoh M, Fujita T, Higo T, Sumida T, Ko T, et al. Cardiomyocyte gene programs encoding morphological and functional signatures in cardiac hypertrophy and failure. Nat Commun. 2018;9:4435. https://doi.org/10.1038/s41467-018-06639-7.
Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148:1145–59. https://doi.org/10.1016/j.cell.2012.02.035.
Nichtova Z, Fernandez-Sanz C, De La Fuente S, Yuan Y, Hurst S, Lanvermann S, et al. Enhanced mitochondria-SR tethering triggers adaptive cardiac muscle remodeling. Circ Res. 2023;132:e171–e187. https://doi.org/10.1161/CIRCRESAHA.122.321833.
MacLean A, Legendre F, Appanna VD. The tricarboxylic acid (TCA) cycle: a malleable metabolic network to counter cellular stress. Crit Rev Biochem Mol Biol. 2023;58:81–97. https://doi.org/10.1080/10409238.2023.2201945.
Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol. 2018;15:387–407. https://doi.org/10.1038/s41569-018-0007-y.
Ritterhoff J, Tian R. Metabolic mechanisms in physiological and pathological cardiac hypertrophy: new paradigms and challenges. Nat Rev Cardiol. 2023;20:812–29. https://doi.org/10.1038/s41569-023-00887-x.
Packer M. Role of deranged energy deprivation signaling in the pathogenesis of cardiac and renal disease in states of perceived nutrient overabundance. Circulation. 2020;141:2095–105. https://doi.org/10.1161/CIRCULATIONAHA.119.045561.
Chakrabarty RP, Chandel NS. Mitochondria as signaling organelles control mammalian stem cell fate. Cell Stem Cell. 2021;28:394–408. https://doi.org/10.1016/j.stem.2021.02.011.
Peace CG, O’Carroll SM, O’Neill LAJ. Fumarate hydratase as a metabolic regulator of immunity. Trends Cell Biol. 2024;34:442–50. https://doi.org/10.1016/j.tcb.2023.10.005.
Reyes C, Karamurzin Y, Frizzell N, Garg K, Nonaka D, Chen YB, et al. Uterine smooth muscle tumors with features suggesting fumarate hydratase aberration: detailed morphologic analysis and correlation with S-(2-succino)-cysteine immunohistochemistry. Mod Pathol. 2014;27:1020–7. https://doi.org/10.1038/modpathol.2013.215.
Ooi A. Advances in hereditary leiomyomatosis and renal cell carcinoma (HLRCC) research. Semin Cancer Biol. 2020;61:158–66. https://doi.org/10.1016/j.semcancer.2019.10.016.
Liang J, Sun G, Pan X, Zhang M, Shen P, Zhu S, et al. Genomic and transcriptomic features between primary and paired metastatic fumarate hydratase-deficient renal cell carcinoma. Genome Med. 2023;15:31 https://doi.org/10.1186/s13073-023-01182-7.
Sullivan LB, Martinez-Garcia E, Nguyen H, Mullen AR, Dufour E, Sudarshan S, et al. The proto-oncometabolite fumarate binds glutathione to amplify ROS-dependent signaling. Mol Cell. 2013;51:236–48. https://doi.org/10.1016/j.molcel.2013.05.003.
Frezza C, Zheng L, Folger O, Rajagopalan KN, MacKenzie ED, Jerby L, et al. Haem oxygenase is synthetically lethal with the tumour suppressor fumarate hydratase. Nature. 2011;477:225–8. https://doi.org/10.1038/nature10363.
Wilde BR, Chakraborty N, Matulionis N, Hernandez S, Ueno D, Gee ME, et al. FH variant pathogenicity promotes purine salvage pathway dependence in kidney cancer. Cancer Discov. 2023;13:2072–89. https://doi.org/10.1158/2159-8290.CD-22-0874.
Torresan F, Iacobone C, Giorgino F, Iacobone M. Genetic and molecular biomarkers in aggressive pheochromocytomas and paragangliomas. Int J Mol Sci. 2024;25. https://doi.org/10.3390/ijms25137142
Yang H, Wu JW, Wang SP, Severi I, Sartini L, Frizzell N, et al. Adipose-specific deficiency of fumarate hydratase in mice protects against obesity, hepatic steatosis, and insulin resistance. Diabetes. 2016;65:3396–409. https://doi.org/10.2337/db16-0136.
Ashrafian H, Czibik G, Bellahcene M, Aksentijević D, Smith AC, Mitchell SJ, et al. Fumarate is cardioprotective via activation of the Nrf2 antioxidant pathway. Cell Metab. 2012;15:361–71. https://doi.org/10.1016/j.cmet.2012.01.017.
Saiyang X, Qingqing W, Man X, Chen L, Min Z, Yun X, et al. Activation of Toll-like receptor 7 provides cardioprotection in septic cardiomyopathy-induced systolic dysfunction. Clin Transl Med. 2021;11:e266 https://doi.org/10.1002/ctm2.266. Jan
Zhang X, Hu C, Ma ZG, Hu M, Yuan XP, Yuan YP, et al. Tisp40 prevents cardiac ischemia/reperfusion injury through the hexosamine biosynthetic pathway in male mice. Nat Commun. 2023;14:3383 https://doi.org/10.1038/s41467-023-39159-0.
Xie S, Deng W, Chen J, Wu QQ, Li H, Wang J. et al. Andrographolide protects against adverse cardiac remodeling after myocardial infarction through enhancing Nrf2 signaling pathway. Int J Biol Sci. 2020;16:12–26. https://doi.org/10.7150/ijbs.37269.
Xie S, Xing Y, Shi W, Zhang M, Chen M, Fang W, et al. Cardiac fibroblast heat shock protein 47 aggravates cardiac fibrosis post myocardial ischemia-reperfusion injury by encouraging ubiquitin specific peptidase 10 dependent Smad4 deubiquitination. Acta Pharm Sin B. 2022;12:4138–53. https://doi.org/10.1016/j.apsb.2022.07.022.
Zhang X, Hu C, Kong CY, Song P, Wu HM, Xu SC, et al. FNDC5 alleviates oxidative stress and cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via activating AKT. Cell Death Differ. 2020;27:540–55. https://doi.org/10.1038/s41418-019-0372-z.
Xie S, Chen M, Fang W, Liu S, Wu Q, Liu C, et al. Diminished arachidonate 5-lipoxygenase perturbs phase separation and transcriptional response of Runx2 to reverse pathological ventricular remodeling. EBioMedicine. 2022;86:104359 https://doi.org/10.1016/j.ebiom.2022.104359.
Xie S, Zhang M, Shi W, Xing Y, Huang Y, Fang WX, et al. Long-term activation of glucagon-like peptide-1 receptor by dulaglutide prevents diabetic heart failure and metabolic remodeling in type 2 diabetes. J Am Heart Assoc. 2022;11:e026728 https://doi.org/10.1161/JAHA.122.026728.
Liu J, Li W, Deng KQ, Tian S, Liu H, Shi H, et al. The E3 ligase TRIM16 is a key suppressor of pathological cardiac hypertrophy. Circ Res. 2022;130:1586–600. https://doi.org/10.1161/CIRCRESAHA.121.318866.
Zhang T, Li L, Mo X, Xie S, Liu S, Zhao N, et al. Matairesinol blunts adverse cardiac remodeling and heart failure induced by pressure overload by regulating Prdx1 and PI3K/AKT/FOXO1 signaling. Phytomedicine. 2024;135:156054 https://doi.org/10.1016/j.phymed.2024.156054.
Yang D, Fan D, Guo Z, Liu FY, Wang MY, An P, et al. SENP1 protects against pressure overload-induced cardiac remodeling and dysfunction via inhibiting STAT3 signaling. J Am Heart Assoc. 2022;11:e027004 https://doi.org/10.1161/JAHA.122.027004.
Ma YL, Kong CY, Guo Z, Wang MY, Wang P, Liu FY, et al. Semaglutide ameliorates cardiac remodeling in male mice by optimizing energy substrate utilization through the Creb5/NR4a1 axis. Nat Commun. 2024;15:4757 https://doi.org/10.1038/s41467-024-48970-2.
Watson WD, Green PG, Lewis AJM, Arvidsson P, De Maria GL, Arheden H, et al. Retained metabolic flexibility of the failing human heart. Circulation. 2023;148:109–23. https://doi.org/10.1161/CIRCULATIONAHA.122.062166.
Tyrakis PA, Yurkovich ME, Sciacovelli M, Papachristou EK, Bridges HR, Gaude E, et al. Fumarate hydratase loss causes combined respiratory chain defects. Cell Rep. 2017;21:1036–47. https://doi.org/10.1016/j.celrep.2017.09.092.
Vercellino I, Sazanov LA. The assembly, regulation and function of the mitochondrial respiratory chain. Nat Rev Mol Cell Biol. 2022;23:141–61. https://doi.org/10.1038/s41580-021-00415-0.
Kuhn AR, van Bilsen M. Oncometabolism: a paradigm for the metabolic remodeling of the failing heart. Int J Mol Sci. 2022;23 https://doi.org/10.3390/ijms232213902.
Fernandez-Caggiano M, Kamynina A, Francois AA, Prysyazhna O, Eykyn TR, Krasemann S, et al. Mitochondrial pyruvate carrier abundance mediates pathological cardiac hypertrophy. Nat Metab. 2020;2:1223–31. https://doi.org/10.1038/s42255-020-00276-5.
Schaffer SW, Shimada-Takaura K, Jong CJ, Ito T, Takahashi K. Impaired energy metabolism of the taurine‑deficient heart. Amino Acids. 2016;48:549–58. https://doi.org/10.1007/s00726-015-2110-2.
Paton CM, Ntambi JM. Biochemical and physiological function of stearoyl-CoA desaturase. Am J Physiol Endocrinol Metab. 2009;297:E28–37. https://doi.org/10.1152/ajpendo.90897.2008.
Guillou H, Zadravec D, Martin PG, Jacobsson A. The key roles of elongases and desaturases in mammalian fatty acid metabolism: Insights from transgenic mice. Prog Lipid Res. 2010;49:186–99. https://doi.org/10.1016/j.plipres.2009.12.002.
Nie L, Pascoa TC, Pike ACW, Bushell SR, Quigley A, Ruda GF, et al. The structural basis of fatty acid elongation by the ELOVL elongases. Nat Struct Mol Biol. 2021;28:512–20. https://doi.org/10.1038/s41594-021-00605-6.
Purdy JG, Shenk T, Rabinowitz JD. Fatty acid elongase 7 catalyzes lipidome remodeling essential for human cytomegalovirus replication. Cell Rep. 2015;10:1375–85. https://doi.org/10.1016/j.celrep.2015.02.003.
Vanani AR, Kalantari H, Mahdavinia M, Rashno M, Khorsandi L, Khodayar MJ. Dimethyl fumarate reduces oxidative stress, inflammation and fat deposition by modulation of Nrf2, SREBP-1c and NF-kappaB signaling in HFD fed mice. Life Sci. 2021;283:119852 https://doi.org/10.1016/j.lfs.2021.119852.
Yang M, Chen X, Zhang J, Xiong E, Wang Q, Fang W, et al. ME2 promotes proneural-mesenchymal transition and lipogenesis in glioblastoma. Front Oncol. 2021;11:715593 https://doi.org/10.3389/fonc.2021.715593
Tallquist MD, Molkentin JD. Redefining the identity of cardiac fibroblasts. Nat Rev Cardiol. 2017;14:484–91. https://doi.org/10.1038/nrcardio.2017.57.
Bertero E, Maack C. Metabolic remodelling in heart failure. Nat Rev Cardiol. 2018;15:457–70. https://doi.org/10.1038/s41569-018-0044-6.
Xie SY, Liu SQ, Zhang T, Shi WK, Xing Y, Fang WX, et al. USP28 serves as a key suppressor of mitochondrial morphofunctional defects and cardiac dysfunction in the diabetic heart. Circulation. 2024;149:684–706. https://doi.org/10.1161/CIRCULATIONAHA.123.065603.
Gibb AA, Hill BG. Metabolic coordination of physiological and pathological cardiac remodeling. Circ Res. 2018;123:107–28. https://doi.org/10.1161/CIRCRESAHA.118.312017.
Lin LC, Liu ZY, Yang JJ, Zhao JY, Tao H. Lipid metabolism reprogramming in cardiac fibrosis. Trends Endocrinol Metab. 2024;35:164–75. https://doi.org/10.1016/j.tem.2023.10.004.
Da Dalt L, Cabodevilla AG, Goldberg IJ, Norata GD. Cardiac lipid metabolism, mitochondrial function, and heart failure. Cardiovasc Res. 2023;119:1905–14. https://doi.org/10.1093/cvr/cvad100.
Linehan WM, Rouault TA. Molecular pathways: fumarate hydratase-deficient kidney cancer-targeting the Warburg effect in cancer. Clin Cancer Res. 2013;19:3345–52. https://doi.org/10.1158/1078-0432.CCR-13-0304.
Hooftman A, Peace CG, Ryan DG, Day EA, Yang M, McGettrick AF, et al. Macrophage fumarate hydratase restrains mtRNA-mediated interferon production. Nature. 2023;615:490–8. https://doi.org/10.1038/s41586-023-05720-6.
Zecchini V, Paupe V, Herranz-Montoya I, Janssen J, Wortel IMN, Morris JL, et al. Fumarate induces vesicular release of mtDNA to drive innate immunity. Nature. 2023;615:499–506. https://doi.org/10.1038/s41586-023-05770-w.
Sciacovelli M, Goncalves E, Johnson TI, Zecchini VR, da Costa AS, Gaude E, et al. Fumarate is an epigenetic modifier that elicits epithelial-to-mesenchymal transition. Nature. 2016;537:544–7. https://doi.org/10.1038/nature19353.
Adam, Ramracheya R J, Chibalina MV, Ternette N, Hamilton A, Tarasov AI, et al. Fumarate hydratase deletion in pancreatic beta cells leads to progressive diabetes. Cell Rep. 2017;20:3135–48. https://doi.org/10.1016/j.celrep.2017.08.093.
Acknowledgements
We thank Home for Researchers editorial team (www.home-for-researchers.com) for language editing service. We thank Baiqu for providing transcriptome sequencing and non-targeted metabolome sequencing.
Funding
The authors receive support from the National Key R&D Program of China (2018YFC1311300); the National Natural Science Foundation of China (Nos: 82170245, 81860080, and 82070204); and the Regional Innovation and Development Joint Fund of National Natural Science Foundation of China (No. U22A20269). China Postdoctoral Science Foundation funded project (2025M772320) and Postdoctoral Innovative Talents Support Program (BX20250257), the Open Project—Seed Fund Project of Hubei Provincial Key Laboratory (2024KFZ021).
Author information
Authors and Affiliations
Contributions
LLL and CJS: designed research; performed research; contributed new reagents; analyzed data and wrote the paper; SYX: Contributed new reagents and analyzed data; XTM, YX, and TZ: Responsible for most of the experiments; WD and YYM: Funding support and final paper guidance and review; Others: Participated in some experiment.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Ll., Sun, Cj., Mo, Xt. et al. Fumarate hydratase ameliorates pressure overload induced cardiac remodeling by controlling Elovl7-mediated biosynthesis of unsaturated fatty acids. Acta Pharmacol Sin 47, 328–343 (2026). https://doi.org/10.1038/s41401-025-01637-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-025-01637-0