Abstract
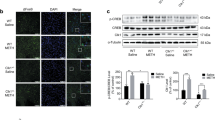
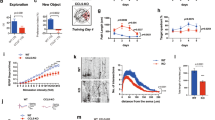
Mitochondria are not only the most important organelles in eukaryotic cells that participate in energy metabolism, signal transduction, cell apoptosis and other physiological processes, but also essential regulators of neurodevelopment, neuroplasticity, survival and adult neurogenesis. The mitochondria-localized hydroxylase Clk-1 is involved in ubiquinone biosynthesis. Recent evidence shows that Clk1+/− mutant mice are resistant to morphine- and methamphetamine-induced conditioned place preference. Given the critical role of learning and memory in drug dependence, we herein explored whether and how Clk1 deficiency affected the cognitive processes in mice. We found that mutant Clk1 mice (Clk1+/−) exhibited recognition memory impairment in novel object recognition (NOR) and novel arm recognition (NAR) tests. In addition, we observed in Clk1+/− mutant mice a selective reduction in dendritic spine density in prefrontal cortex (PFC) but not in the hippocampus (HIP). The expression of brain-derived neurotrophic factor (BDNF) was also decreased in PFC but not in HIP. Furthermore, Clk1+/− mutant mice displayed impairment in the ERK/CREB signaling pathway in PFC that might underlie Clk1+/− mutation-induced changes in BDNF and dendritic morphology. Administration of antipsychotic drugs aripiprazole (0.3 mg·kg−1·d−1, i.p.) or risperidone (1 mg·kg−1·d−1, i.p.) for 7 days fully rescued Clk1 mutation-induced recognition memory deficits. This study provides primary evidence highlighting the role of mitochondrial Clk1 in the regulation of recognition memory and presents an informative model for investigating mitochondrial function in learning and memory.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Goldman JG, Sieg E. Cognitive impairment and dementia in parkinson disease. Clin Geriatr Med. 2020;36:365–77.
McCutcheon RA, Keefe RSE, McGuire PK. Cognitive impairment in schizophrenia: aetiology, pathophysiology, and treatment. Mol Psychiatry. 2023;28:1902–18.
Friedman NP, Robbins TW. The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology. 2022;47:72–89.
Arion D, Huo Z, Enwright JF, Corradi JP, Tseng G, Lewis DA. Transcriptome alterations in prefrontal pyramidal cells distinguish schizophrenia from bipolar and major depressive disorders. Biol Psychiatry. 2017;82:594–600.
Yan Z, Rein B. Mechanisms of synaptic transmission dysregulation in the prefrontal cortex: pathophysiological implications. Mol Psychiatry. 2022;27:445–65.
Acosta MJ, Vazquez Fonseca L, Desbats MA, Cerqua C, Zordan R, Trevisson E, et al. Coenzyme q biosynthesis in health and disease. Biochim Biophys Acta. 2016;1857:1079–85.
Annesley SJ, Fisher PR. Mitochondria in health and disease. Cells. 2019;8:680.
Nunnari J, Suomalainen A. Mitochondria: In sickness and in health. Cell. 2012;148:1145–59.
Kim Y, Vadodaria KC, Lenkei Z, Kato T, Gage FH, Marchetto MC, et al. Mitochondria, metabolism, and redox mechanisms in psychiatric disorders. Antioxid Redox Signal. 2019;31:275–317.
Khacho M, Harris R, Slack RS. Mitochondria as central regulators of neural stem cell fate and cognitive function. Nat Rev Neurosci. 2019;20:34–48.
Singh A, Kukreti R, Saso L, Kukreti S. Oxidative stress: a key modulator in neurodegenerative diseases. Molecules. 2019;24:1583.
Yan MH, Wang X, Zhu X. Mitochondrial defects and oxidative stress in alzheimer disease and parkinson disease. Free Radic Biol Med. 2013;62:90–101.
Peruzzotti Jametti L, Willis CM, Krzak G, Hamel R, Pirvan L, Ionescu RB, et al. Mitochondrial complex I activity in microglia sustains neuroinflammation. Nature. 2024;628:195–203.
Li Y, Xia X, Wang Y, Zheng JC. Mitochondrial dysfunction in microglia: a novel perspective for pathogenesis of alzheimer’s disease. J Neuroinflammation. 2022;19:248.
Bajwa E, Pointer CB, Klegeris A. The role of mitochondrial damage-associated molecular patterns in chronic neuroinflammation. Mediat Inflamm. 2019;2019:11.
He CH, Xie LX, Allan CM, Tran UC, Clarke CF. Coenzyme q supplementation or over-expression of the yeast coq8 putative kinase stabilizes multi-subunit coq polypeptide complexes in yeast coq null mutants. Biochim Biophys Acta. 2014;1841:630–44.
Monaghan RM, Barnes RG, Fisher K, Andreou T, Rooney N, Poulin GB, et al. A nuclear role for the respiratory enzyme clk-1 in regulating mitochondrial stress responses and longevity. Nat Cell Biol. 2015;17:782–92.
Wang Y, Branicky R, Stepanyan Z, Carroll M, Guimond MP, Hihi A, et al. The anti-neurodegeneration drug clioquinol inhibits the aging-associated protein Clk-1. J Biol Chem. 2009;284:314–23.
Zheng H, Lapointe J, Hekimi S. Lifelong protection from global cerebral ischemia and reperfusion in long-lived Mclk1+/- mutants. Exp Neurol. 2010;223:557–65.
Gu R, Zhang F, Chen G, Han C, Liu J, Ren Z, et al. Clk1 deficiency promotes neuroinflammation and subsequent dopaminergic cell death through regulation of microglial metabolic reprogramming. Brain Behav Immun. 2017;60:206–19.
Yan Q, Han C, Wang G, Waddington JL, Zheng L, Zhen X. Activation of ampk/mtorc1-mediated autophagy by metformin reverses clk1 deficiency-sensitized dopaminergic neuronal death. Mol Pharmacol. 2017;92:640–52.
Yan PJ, Ren ZX, Shi ZF, Wan CL, Han CJ, Zhu LS, et al. Dysregulation of iron homeostasis and methamphetamine reward behaviors in clk1-deficient mice. Acta Pharmacol Sin. 2022;43:1686–98.
Rogoz Z, Wasik A, Lorenc-Koci E. Combined treatment with aripiprazole and antidepressants reversed some MK-801-induced schizophrenia-like symptoms in mice. Pharmacol Rep. 2018;70:623–30.
Wu L, Feng X, Li T, Sun B, Khan MZ, He L. Risperidone ameliorated Abeta(1-42)-induced cognitive and hippocampal synaptic impairments in mice. Behav Brain Res. 2017;322:145–56.
Lu C, Li S, Kang L, Li Q, Chen H, Lin Y, et al. Aripiprazole combined with nerve growth factor improves cognitive function in mice with schizophrenia model. Neurosci Lett. 2023;812:137410.
Chen J, Li G, Qin P, Chen J, Ye N, Waddington JL, et al. Allosteric modulation of the sigma-1 receptor elicits antipsychotic-like effects. Schizophr Bull. 2022;48:474–84.
Wiatrak B, Kubis Kubiak A, Piwowar A, Barg E. PC12 cell line: cell types, coating of culture vessels, differentiation and other culture conditions. Cells. 2020;9:958.
Ren ZX, Zhao YF, Cao T, Zhen XC. Dihydromyricetin protects neurons in an mptp-induced model of Parkinson’s disease by suppressing glycogen synthase kinase-3 beta activity. Acta Pharmacol Sin. 2016;37:1315–24.
Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–45.
Mouri A, Lee HJ, Mamiya T, Aoyama Y, Matsumoto Y, Kubota H, et al. Hispidulin attenuates the social withdrawal in isolated disrupted-in-schizophrenia-1 mutant and chronic phencyclidine-treated mice. Br J Pharmacol. 2020;177:3210–24.
Kaminski J, Rutishauser U. Between persistently active and activity-silent frameworks: novel vistas on the cellular basis of working memory. Ann N Y Acad Sci. 2020;1464:64–75.
Arion D, Enwright JF 3rd, Gonzalez-Burgos G, Lewis DA. Cell type-specific profiles and developmental trajectories of transcriptomes in primate prefrontal layer 3 pyramidal neurons: implications for schizophrenia. Am J Psychiatry. 2024;181:920–34.
Li S, Ma C, Li Y, Chen R, Liu Y, Wan LP, et al. The schizophrenia-associated missense variant rs13107325 regulates dendritic spine density. Transl Psychiatry. 2022;12:361.
Yao W, Zhou P, Yan Q, Wu X, Xia Y, Li W, et al. ERVWE1 reduces hippocampal neuron density and impairs dendritic spine morphology through inhibiting Wnt/JNK non-canonical pathway via miR-141-3p in schizophrenia. Viruses. 2023;15:168.
Kellner Y, Godecke N, Dierkes T, Thieme N, Zagrebelsky M, Korte M. The BDNF effects on dendritic spines of mature hippocampal neurons depend on neuronal activity. Front Synaptic Neurosci. 2014;6:5.
Lv J, Lu C, Jiang N, Wang H, Huang H, Chen Y, et al. Protective effect of ginsenoside Rh2 on scopolamine-induced memory deficits through regulation of cholinergic transmission, oxidative stress and the erk-creb-bdnf signaling pathway. Phytother Res. 2021;35:337–45.
Yu X, Guan Q, Wang Y, Shen H, Zhai L, Lu X, et al. Anticonvulsant and anti-apoptosis effects of salvianolic acid B on pentylenetetrazole-kindled rats via Akt/CREB/BDNF signaling. Epilepsy Res. 2019;154:90–6.
Yoshii A, Constantine Paton M. BDNF induces transport of psd-95 to dendrites through pi3k-akt signaling after nmda receptor activation. Nat Neurosci. 2007;10:702–11.
Ray MT, Shannon Weickert C, Webster MJ. Decreased bdnf and trkb mrna expression in multiple cortical areas of patients with schizophrenia and mood disorders. Transl Psychiatry. 2014;4:e389.
Szota AM, Kowalewska B, Cwiklinska Jurkowska M, Drozdz W. The Influence of electroconvulsive therapy (ECT) on brain-derived neurotrophic factor (BDNF) plasma level in patients with schizophrenia-a systematic review and meta-analysis. J Clin Med. 2023;12:5728.
Mohammadi A, Rashidi E, Amooeian VG. Brain, blood, cerebrospinal fluid, and serum biomarkers in schizophrenia. Psychiatry Res. 2018;265:25–38.
Angelucci F, Brene S, Mathe AA. BDNF in schizophrenia, depression and corresponding animal models. Mol Psychiatry. 2005;10:345–52.
Jin H, Yang C, Jiang C, Li L, Pan M, Li D, et al. Evaluation of neurotoxicity in BALB/c mice following chronic exposure to polystyrene microplastics. Environ Health Perspect. 2022;130:107002.
Luine V, Mohan G, Attalla S, Jacome L, Frankfurt M. Androgens enhance recognition memory and dendritic spine density in the hippocampus and prefrontal cortex of ovariectomized female rats. Neuroscience. 2025;568:465–75.
Diab AM, Wigerius M, Quinn DP, Qi J, Shahin I, Paffile J, et al. Nck1 modulates neuronal actin dynamics and promotes dendritic spine, synapse, and memory formation. J Neurosci. 2023;43:885–901.
Cavalcante LES, Zinn CG, Schmidt SD, Saenger BF, Ferreira FF, Furini CRG, et al. Modulation of the storage of social recognition memory by neurotransmitter systems in the insular cortex. Behav Brain Res. 2017;334:129–34.
Borkar CD, Bharne AP, Nagalakshmi B, Sakharkar AJ, Subhedar NK, Kokare DM. Cocaine- and amphetamine-regulated transcript peptide (CART) alleviates MK-801-induced schizophrenic dementia-like symptoms. Neuroscience. 2018;375:94–107.
Perez Palomar B, Erdozain AM, Erkizia Santamaria I, Ortega JE, Meana JJ. Maternal immune activation induces cortical catecholaminergic hypofunction and cognitive impairments in offspring. J Neuroimmune Pharmacol. 2023;18:348–65.
Steimel SA, Meisenhelter S, Quon RJ, Camp EJ, Tom R, Bujarski KA, et al. Accelerated long-term forgetting of recall and recognition memory in people with epilepsy. Epilepsy Behav. 2023;141:109152.
Sanborn V, Putcha D, Tremont G. Correlates of recognition memory performance in amnestic mild cognitive impairment. J Clin Exp Neuropsychol. 2018;40:205–11.
Zhang Y, Liu X, Wiggins KL, Kurniansyah N, Guo X, Rodrigue AL, et al. Association of mitochondrial dna copy number with brain mri markers and cognitive function: a meta-analysis of community-based cohorts. Neurology. 2023;100:e1930–e43.
Alexander JF, Seua AV, Arroyo LD, Ray PR, Wangzhou A, Heibeta-Luckemann L, et al. Nasal administration of mitochondria reverses chemotherapy-induced cognitive deficits. Theranostics. 2021;11:3109–30.
Hara Y, Yuk F, Puri R, Janssen WG, Rapp PR, Morrison JH. Presynaptic mitochondrial morphology in monkey prefrontal cortex correlates with working memory and is improved with estrogen treatment. Proc Natl Acad Sci USA. 2014;111:486–91.
Picard M, McEwen BS. Mitochondria impact brain function and cognition. Proc Natl Acad Sci USA. 2014;111:7–8.
Cheng J, Yang HL, Gu CJ, Liu YK, Shao J, Zhu R, et al. Melatonin restricts the viability and angiogenesis of vascular endothelial cells by suppressing HIF-1alpha/ROS/VEGF. Int J Mol Med. 2019;43:945–55.
Heroux NA, Horgan CJ, Pinizzotto CC, Rosen JB, Stanton ME. Medial prefrontal and ventral hippocampal contributions to incidental context learning and memory in adolescent rats. Neurobiol Learn Mem. 2019;166:107091.
Chao OY, Nikolaus S, Yang YM, Huston JP. Neuronal circuitry for recognition memory of object and place in rodent models. Neurosci Biobehav Rev. 2022;141:104855.
Zarneshan SN, Fakhri S, Khan H. Targeting AKT/CREB/BDNF signaling pathway by ginsenosides in neurodegenerative diseases: a mechanistic approach. Pharmacol Res. 2022;177:106099.
Coley AA, Gao WJ. PSD-95 deficiency disrupts pfc-associated function and behavior during neurodevelopment. Sci Rep. 2019;9:9486.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2021YFE0206000) and a National Innovation of Science and Technology-2030 (Program of Brain Science and Brain-Inspired Intelligence Technology) Grant (2021ZD0204004). We also acknowledge support from the Suzhou International Academician Work Station and Priority Academic Program Development (PAPD) of the Jiangsu Higher Education Institutes.
Author information
Authors and Affiliations
Contributions
XCZ designed the research. ZFS analyzed the data and drafted the manuscript. ZFS, ZXY and ZXM performed the behavioral, molecular biological and biochemical experiments. QBC, LBW and LHG performed experiments and collected data. JLW and XCZ analyzed and interpreted the data and edited the manuscript. All authors reviewed the manuscript and approved the submission for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, Zf., Yu, Zx., Gu, Lh. et al. Selective recognition memory impairment in mitochondrial hydroxylase Clk1 mutant mice, rescued by antipsychotics. Acta Pharmacol Sin (2025). https://doi.org/10.1038/s41401-025-01641-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41401-025-01641-4