Abstract
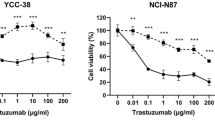
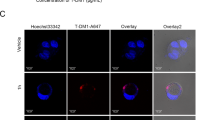
The anti-HER2 antibody‒drug conjugate (ADC) DS-8201 presents new hope for patients with advanced HER2-positive tumors. Its clinical application, however, is hindered by serious adverse reactions and reduced efficacy following long-term treatment. In this study, we investigated the factors influencing the sensitivity of DS-8201 and developed effective combination regimens to optimize its therapeutic efficacy. We showed that HER3 upregulation diminished the sensitivity of HER2-positive tumor cells to DS-8201. We found that DS-8201 treatment activated DNA damage repair responses in BT-474 cells, in which the ATR kinase pathway induced the expression of the HER3 transcription factor FoxO1, leading to increased HER3 levels. This process was triggered by the payload component of DS-8201, the topoisomerase I inhibitor DXd, rather than the antibody. Based on this finding, we showed that combining DS-8201 with either a HER3-targeting antibody (SIBP-03) or an ATR inhibitor (BAY1895344) resulted in significant synergistic antitumor efficacy without substantial toxicity in vitro or in vivo. Overall, this study revealed that the ATR/FoxO1/HER3 pathway plays a critical role in modulating the efficacy of DS-8201, suggesting that combining DS-8201 with ATR or HER3 inhibition represents a promising therapeutic strategy for HER2-positive cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Sheng Q, Liu J. The therapeutic potential of targeting the EGFR family in epithelial ovarian cancer. Br J Cancer. 2011;104:1241–5.
Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997;16:1647–55.
Roskoski R Jr. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol Res. 2014;79:34–74.
Oh DY, Bang YJ. HER2-targeted therapies - a role beyond breast cancer. Nat Rev Clin Oncol. 2020;17:33–48.
Sidaway P. T-DXd is effective after T-DM1. Nat Rev Clin Oncol. 2023;20:426.
Nakada T, Sugihara K, Jikoh T, Abe Y, Agatsuma T. The latest research and development into the antibody-drug conjugate, [fam-] trastuzumab deruxtecan (DS-8201a), for HER2 cancer therapy. Chem Pharm Bull (Tokyo). 2019;67:173–85.
Yver A, Agatsuma T, Soria JC. The art of innovation: clinical development of trastuzumab deruxtecan and redefining how antibody-drug conjugates target HER2-positive cancers. Ann Oncol. 2020;31:430–4.
Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387:9–20.
Li BT, Smit EF, Goto Y, Nakagawa K, Udagawa H, Mazières J, et al. Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. N Engl J Med. 2022;386:241–51.
Doi T, Shitara K, Naito Y, Shimomura A, Fujiwara Y, Yonemori K, et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol. 2017;18:1512–22.
Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382:610–21.
Papa F, Grinda T, Rassy E, Cheickh-Hussin R, Ribeiro J, Antonuzzo L, et al. Long road towards effective HER3 targeting in breast cancer. Cancer Treat Rev. 2024;129:102786.
Pan L, Li J, Xu Q, Gao Z, Yang M, Wu X, et al. HER2/PI3K/AKT pathway in HER2-positive breast cancer: A review. Med (Balt). 2024;103:e38508.
Vaught DB, Stanford JC, Young C, Hicks DJ, Wheeler F, Rinehart C, et al. HER3 is required for HER2-induced preneoplastic changes to the breast epithelium and tumor formation. Cancer Res. 2012;72:2672–82.
Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP, et al. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2008;68:5878–87.
Watanabe S, Yonesaka K, Tanizaki J, Nonagase Y, Takegawa N, Haratani K, et al. Targeting of the HER2/HER3 signaling axis overcomes ligand-mediated resistance to trastuzumab in HER2-positive breast cancer. Cancer Med. 2019;8:1258–68.
Chen X, Liu P, Wang Q, Li Y, Fu L, Fu H, et al. DCZ3112, a novel Hsp90 inhibitor, exerts potent antitumor activity against HER2-positive breast cancer through disruption of Hsp90-Cdc37 interaction. Cancer Lett. 2018;434:70–80.
Li WJ, Xie CY, Zhu X, Tang J, Wang L, Lou LG. SIBP-03, a novel anti-HER3 antibody, exerts antitumor effects and synergizes with EGFR- and HER2-targeted drugs. Acta Pharmacol Sin. 2024;45:857–66.
Kumagai S, Koyama S, Nishikawa H. Antitumour immunity regulated by aberrant ERBB family signalling. Nat Rev Cancer. 2021;21:181–97.
Yonesaka K, Tanizaki J, Maenishi O, Haratani K, Kawakami H, Tanaka K, et al. HER3 augmentation via blockade of EGFR/AKT signaling enhances anticancer activity of HER3-targeting patritumab deruxtecan in EGFR-mutated non-small cell lung cancer. Clin Cancer Res. 2022;28:390–403.
Hanker AB, Brown BP, Meiler J, Marín A, Jayanthan HS, Ye D, et al. Co-occurring gain-of-function mutations in HER2 and HER3 modulate HER2/HER3 activation, oncogenesis, and HER2 inhibitor sensitivity. Cancer Cell. 2021;39:1099–1114.e8.
Cervantes-Gomez F, Nimmanapalli R, Gandhi V. Transcription inhibition of heat shock proteins: a strategy for combination of 17-allylamino-17-demethoxygeldanamycin and actinomycin d. Cancer Res. 2009;69:3947–54.
Sobell HM. Actinomycin and DNA transcription. Proc Natl Acad Sci USA. 1985;82:5328–31.
Takahashi S, Karayama M, Takahashi M, Watanabe J, Minami H, Yamamoto N, et al. Pharmacokinetics, safety, and efficacy of trastuzumab deruxtecan with concomitant ritonavir or itraconazole in patients with HER2-expressing advanced solid tumors. Clin Cancer Res. 2021;27:5771–80.
Tsurutani J, Iwata H, Krop I, Jänne PA, Doi T, Takahashi S, et al. Targeting HER2 with trastuzumab deruxtecan: a dose-expansion, phase I study in multiple advanced solid tumors. Cancer Discov 2020;10:1078. Corrected and republished from: Cancer Discov. 2020; 10: 688-701.
Pilié PG, Tang C, Mills GB, Yap TA. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat Rev Clin Oncol. 2019;16:81–104.
Lee HJ, Lan L, Peng G, Chang WC, Hsu MC, Wang YN, et al. Tyrosine 370 phosphorylation of ATM positively regulates DNA damage response. Cell Res. 2015;25:225–36.
Liccardi G, Hartley JA, Hochhauser D. EGFR nuclear translocation modulates DNA repair following cisplatin and ionizing radiation treatment. Cancer Res. 2011;71:1103–14.
Yan Y, Hein AL, Greer PM, Wang Z, Kolb RH, Batra SK, et al. A novel function of HER2/Neu in the activation of G2/M checkpoint in response to γ-irradiation. Oncogene. 2015;34:2215–26.
Francis DM, Huang S, Armstrong EA, Werner LR, Hullett C, Li C, et al. Pan-HER inhibitor augments radiation response in human lung and head and neck cancer models. Clin Cancer Res. 2016;22:633–43.
Tan L, Zhang J, Wang Y, Wang X, Wang Y, Zhang Z, et al. Development of dual inhibitors targeting epidermal growth factor receptor in cancer therapy. J Med Chem. 2022;65:5149–83.
Austin CD, De Mazière AM, Pisacane PI, van Dijk SM, Eigenbrot C, Sliwkowski MX, et al. Endocytosis and sorting of ErbB2 and the site of action of cancer therapeutics trastuzumab and geldanamycin. Mol Biol Cell. 2004;15:5268–82.
Narayan M, Wilken JA, Harris LN, Baron AT, Kimbler KD, Maihle NJ. Trastuzumab-induced HER reprogramming in “resistant” breast carcinoma cells. Cancer Res. 2009;69:2191–4.
Gijsen M, King P, Perera T, Parker PJ, Harris AL, Larijani B, et al. HER2 phosphorylation is maintained by a PKB negative feedback loop in response to anti-HER2 herceptin in breast cancer. PLoS Biol. 2016;14:e1002414. Corrected and republished from: PLoS Biol. 2010;8:e1000563.
Schwarz LJ, Hutchinson KE, Rexer BN, Estrada MV, Gonzalez Ericsson PI, Sanders ME, et al. An ERBB1-3 neutralizing antibody mixture with high activity against drug-resistant HER2+ breast cancers with ERBB ligand overexpression. J Natl Cancer Inst. 2017;109:djx065.
Dent RA, Cescon DW, Bachelot T, Jung KH, Shao ZM, Saji S, et al. TROPION-Breast02: datopotamab deruxtecan for locally recurrent inoperable or metastatic triple-negative breast cancer. Future Oncol. 2023;19:2349–59.
Okamoto I, Kuyama S, Girard N, Lu S, Franke F, Li Z, et al. TROPION-Lung07: phase III study of Dato-DXd + pembrolizumab ± platinum-based chemotherapy as 1L therapy for advanced non-small-cell lung cancer. Future Oncol. 2024;20:2927–36.
Jänne PA, Baik C, Su WC, Johnson ML, Hayashi H, Nishio M, et al. Efficacy and safety of patritumab deruxtecan (HER3-DXd) in EGFR inhibitor-resistant, EGFR-mutated non-small cell lung cancer. Cancer Discov. 2022;12:74–89.
Yu HA, Baik C, Kim DW, Johnson ML, Hayashi H, Nishio M, et al. Translational insights and overall survival in the U31402-A-U102 study of patritumab deruxtecan (HER3-DXd) in EGFR-mutated NSCLC. Ann Oncol. 2024;35:437–47.
Suzuki H, Nagase S, Saito C, Takatsuka A, Nagata M, Honda K, et al. Raludotatug deruxtecan, a CDH6-targeting antibody-drug conjugate with a DNA topoisomerase I inhibitor DXd, is efficacious in human ovarian and kidney cancer models. Mol Cancer Ther. 2024;23:257–71.
Trinder A, Ding K, Zhang J. The therapeutic significance of HER3 in non-small cell lung cancer (NSCLC): a review study. Curr Med Chem. 2025;32:434–46.
Zhou KI, Strickler JH, Chen H. Targeting Claudin-18.2 for cancer therapy: updates from 2024 ASCO annual meeting. J Hematol Oncol. 2024;17:73.
Bardia A, Mayer IA, Vahdat LT, Tolaney SM, Isakoff SJ, Diamond JR, et al. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med. 2019;380:741–51.
Dormann C. Metastatic human epidermal growth factor receptor 2-positive breast cancer: current treatment standards and future perspectives. Breast Care (Basel). 2020;15:570–8.
Crystal RG, Gadek JE, Ferrans VJ, Fulmer JD, Line BR, Hunninghake GW. Interstitial lung disease: current concepts of pathogenesis, staging and therapy. Am J Med. 1981;70:542–68.
Tarantino P, Modi S, Tolaney SM, Cortés J, Hamilton EP, Kim SB, et al. Interstitial lung disease induced by anti-ERBB2 antibody-drug conjugates: A review. JAMA Oncol. 2021;7:1873–81.
Kumagai K, Aida T, Tsuchiya Y, Kishino Y, Kai K, Mori K. Interstitial pneumonitis related to trastuzumab deruxtecan, a human epidermal growth factor receptor 2-targeting Ab-drug conjugate, in monkeys. Cancer Sci. 2020;111:4636–45.
Blackford AN, Jackson SP. ATM, ATR, and DNA-PK: the trinity at the heart of the DNA damage response. Mol Cell. 2017;66:801–17.
Zhang C, Chen L, Xie C, Wang F, Wang J, Zhou H, et al. YTHDC1 delays cellular senescence and pulmonary fibrosis by activating ATR in an m6A-independent manner. EMBO J. 2024;43:61–86.
Heumann TR, Stockton S, Cecchini M, Aljumaily R, Shyr C, Whisenant J, et al. A phase I study of irinotecan combined with BAY1895344 (ATR inhibitor) in advanced solid tumors: Results of ETCTN 10402 dose escalation. J Clin Oncol. 2024;42:3077.
Stockton S, Shyr C, Cecchini M, Aljumaily R, Halfdanarson TR, Sonbol MB, et al. A phase I study of ATR inhibitor BAY1895344 (elimusertib) plus topotecan (ETCTN 10402): results of dose escalation. J Clin Oncol. 2024;42:3076.
Takahashi N, Hao Z, Villaruz LC, Zhang J, Ruiz J, Petty WJ, et al. Berzosertib plus topotecan vs topotecan alone in patients with relapsed small cell lung cancer: a randomized clinical trial. JAMA Oncol. 2023;9:1669–77.
Acknowledgements
This research was supported by grants from the Science and Technology Commission of Shanghai Municipality (grant No. 18DZ2293200). The graphical abstract in this manuscript was created with BioGDP.com.
Author information
Authors and Affiliations
Contributions
LGL conceived and designed the research. WJL performed experiments and prepared the figures and manuscript. KGK and YXZ performed part of the cell experiments. WJL, KGK, YXZ, and XXZ contributed to the acquisition and analysis of the data. XZ, JT, and YPL helped with the data analysis and validation. HYF and QY participated in the mouse experiments. LW and LGL revised and approved the manuscript. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Wj., Kang, Kg., Zhang, Yx. et al. HER3 upregulation reduces DS-8201 sensitivity in HER2-positive tumor cells by ATR/CHK1/FoxO1 signaling cascade. Acta Pharmacol Sin (2025). https://doi.org/10.1038/s41401-025-01647-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41401-025-01647-y