Abstract
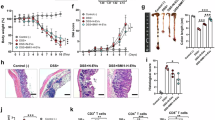
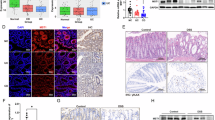
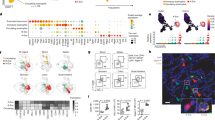
Enteric glial cells (EGCs) play an important role in the pathogenesis of irritable bowel syndrome (IBS). Phosphodiesterase-4 (PDE4) functions as a catalyzing enzyme targeting hydrolyzation of intracellular cyclic adenosine monophosphate (cAMP). Increased PDE4 activity promotes excessive production of pro-inflammatory cytokines and chemokines in various immune and epithelial cells, exacerbating immune cell activation and infiltration in inflamed tissues, inhibition of PDE4 has been proven to be an important strategy for inflammatory and autoimmune diseases. In this study we investigated the pathological role of PDE4 and the therapeutic effects of a PDE4 inhibitor apremilast in IBS. 2,4,6-Trinitrobenzenesulfonic acid (TNBS)-induced IBS model was established in mice, the mice were treated with apremilast (50 mg/kg, i.g.) for 7 days. After treatment, the intestinal motility and visceral sensitivity were assessed. At the end of the study, the mice were euthanized and the blood and colon tissues were collected for analyses. We showed that apremilast treatment significantly ameliorated IBS symptoms in the mice, evidenced by improvement on delayed intestinal motility and visceral hypersensitivity. We found that EGCs were activated in the colon of IBS mice. We then demonstrated that apremilast (10 μM) significantly suppressed TNF-α/IFN-γ stimulated activation of rat EGC cell line CRL-2690 and primary EGCs in vitro, as well as the secretion of EGCs-derived pain mediators and inflammatory factors while ameliorating oxidative stress. These effects depended on the activation of the nuclear factor erythroid 2-related factor 2 (Nrf-2) signaling pathway, which was validated in Nrf-2 knockout EGCs. These results suggest that inhibition of PDE4 by apremilast suppresses EGCs activation by activating the Nrf-2 signaling pathway, leading to decreased expression of pain mediators and inflammatory factors while ameliorating oxidative stress, ultimately alleviating IBS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Saha L. Irritable bowel syndrome: Pathogenesis, diagnosis, treatment, and evidence-based medicine. World J Gastroenterol. 2014;20:6759–73.
Black CJ, Ford AC. Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat Rev Gastroenterol Hepatol. 2020;17:473–86.
Huang KY, Wang FY, Lv M, Ma XX, Tang XD, Lv L. Irritable bowel syndrome: epidemiology, overlap disorders, pathophysiology and treatment. World J Gastroenterol. 2023;29:4120–35.
Holtmann GJ, Ford AC, Talley NJ. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol Hepatol. 2016;1:133–46.
Aguilera-Lizarraga J, Hussein H, Boeckxstaens GE. Immune activation in irritable bowel syndrome: what is the evidence? Nat Rev Immunol. 2022;22:674–86.
Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. 2015;313:949–58.
Enck P, Aziz Q, Barbara G, Farmer AD, Fukudo S, Mayer EA, et al. Irritable bowel syndrome. Nat Rev Dis Prim. 2016;2:16014.
Harvey JM, Sibelli A, Chalder T, Everitt H, Moss-Morris R, Bishop FL. Desperately seeking a cure: Treatment seeking and appraisal in irritable bowel syndrome. Br J Health Psychol. 2018;23:561–79.
Mete R, Tulubas F, Oran M, Yılmaz A, Avci BA, Yildiz K, et al. The role of oxidants and reactive nitrogen species in irritable bowel syndrome: a potential etiological explanation. Med Sci Monit. 2013;19:762–6.
Choghakhori R, Abbasnezhad A, Hasanvand A, Amani R. Inflammatory cytokines and oxidative stress biomarkers in irritable bowel syndrome: Association with digestive symptoms and quality of life. Cytokine. 2017;93:34–43.
Jia H, Zhang Y, Si X, Jin Y, Jiang D, Dai Z, et al. Quercetin alleviates oxidative damage by activating nuclear factor erythroid 2-related factor 2 signaling in porcine enterocytes. Nutrients. 2021;13:375.
Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. 2014;94:329–54.
Camilleri M, Madsen K, Spiller R, Greenwood-Van Meerveld B, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil. 2012;24:503–12.
Xiong Y, Wei H, Chen C, Jiao L, Zhang J, Tan Y, et al. Coptisine attenuates post‑infectious IBS via Nrf2‑dependent inhibition of the NLPR3 inflammasome. Mol Med Rep. 2022;26:362.
Zeng L, Li K, Wei H, Hu J, Jiao L, Yu S, et al. A novel ephA2 inhibitor exerts beneficial effects in PI-IBS in vivo and in vitro models via Nrf2 and NF-κB signaling pathways. Front Pharmacol. 2018;9:272.
Xia Y, Tan W, Yuan F, Lin M, Luo H. Luteolin attenuates oxidative stress and colonic hypermobility in water avoidance stress rats by activating the Nrf2 signaling pathway. Mol Nutr Food Res. 2024;68:e2300126.
Liu S, Pi J, Zhang Q. Signal amplification in the KEAP1-NRF2-ARE antioxidant response pathway. Redox Biol. 2022;54:102389.
Sies H, Berndt C, Jones DP. Oxidative stress. Annu Rev Biochem. 2017;86:715–48.
Linan-Rico A, Ochoa-Cortes F, Schneider R, Christofi FL. Mini-review: Enteric glial cell reactions to inflammation and potential therapeutic implications for GI diseases, motility disorders, and abdominal pain. Neurosci Lett. 2023;812:137395.
Morales-Soto W, Gulbransen BD. Enteric glia: A new player in abdominal pain. Cell Mol Gastroenterol Hepatol. 2019;7:433–45.
Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–84.
Ji RR, Chamessian A, Zhang YQ. Pain regulation by non-neuronal cells and inflammation. Science. 2016;354:572–7.
Ji RR, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain. 2013;154:S10–s28.
Benvenuti L, D’Antongiovanni V, Pellegrini C, Antonioli L, Bernardini N, Blandizzi C, et al. Enteric glia at the crossroads between intestinal immune system and epithelial barrier: implications for parkinson disease. Int J Mol Sci. 2020;21:9199.
Kimono D, Sarkar S, Albadrani M, Seth R, Bose D, Mondal A, et al. Dysbiosis-associated enteric glial cell immune-activation and redox imbalance modulate tight junction protein expression in gulf war illness pathology. Front Physiol. 2019;10:1229.
Capoccia E, Cirillo C, Gigli S, Pesce M, D’Alessandro A, Cuomo R, et al. Enteric glia: a new player in inflammatory bowel diseases. Int J Immunopathol Pharmacol. 2015;28:443–51.
Meira de‐Faria F, Casado‐Bedmar M, Mårten Lindqvist C, Jones MP, Walter SA, Keita ÅV. Altered interaction between enteric glial cells and mast cells in the colon of women with irritable bowel syndrome. Neurogastroenterol Motil. 2021;33:e14130.
Spadaccini M, D’Alessio S, Peyrin-Biroulet L, Danese S. PDE4 inhibition and inflammatory bowel disease: a novel therapeutic avenue. Int J Mol Sci. 2017;18:1276.
Schafer PH, Truzzi F, Parton A, Wu L, Kosek J, Zhang LH, et al. Phosphodiesterase 4 in inflammatory diseases: effects of apremilast in psoriatic blood and in dermal myofibroblasts through the PDE4/CD271 complex. Cell Signal. 2016;28:753–63.
Li H, Zuo J, Tang W. Phosphodiesterase-4 inhibitors for the treatment of inflammatory diseases. Front Pharmacol. 2018;9:1048.
Yang Z, Grinchuk V, Ip SP, Che CT, Fong HH, Lao L, et al. Anti-inflammatory activities of a chinese herbal formula IBS-20 in vitro and in vivo. Evid Based Complement Altern Med. 2012;2012:491496.
Jang JH, Jang SY, Ahn S, Oh JY, Yeom M, Ko SJ, et al. Chronic gut inflammation and dysbiosis in IBS: Unraveling their contribution to atopic dermatitis progression. Int J Mol Sci. 2024;25:2573.
Ke W, Wang Y, Huang S, Liu S, Zhu H, Xie X, et al. Paeoniflorin alleviates inflammatory response in IBS-D mouse model via downregulation of the NLRP3 inflammasome pathway with involvement of miR-29a. Heliyon. 2022;8:e12312.
Jin X, Hu Y, Lin T, Gao F, Xu Z, Hou X, et al. Selenium-enriched bifidobacterium longum DD98 relieves irritable bowel syndrome induced by chronic unpredictable mild stress in mice. Food Funct. 2023;14:5355–74.
Hayeeawaema F, Wichienchot S, Khuituan P. Amelioration of gut dysbiosis and gastrointestinal motility by konjac oligo-glucomannan on loperamide-induced constipation in mice. Nutrition. 2020;73:110715.
Laird JMA, Martinez-Caro L, Garcia-Nicas E, Cervero F. A new model of visceral pain and referred hyperalgesia in the mouse. Pain. 2001;92:335–42.
Wirtz S, Popp V, Kindermann M, Gerlach K, Weigmann B, Fichtner-Feigl S, et al. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat Protoc. 2017;12:1295–309.
Teramoto H, Hirashima N, Tanaka M. A simple method for purified primary culture of enteric glial cells from mouse small intestine. Biol Pharm Bull. 2022;45:547–51.
Wang Z, Ocadiz-Ruiz R, Sundaresan S, Ding L, Hayes M, Sahoo N, et al. Isolation of enteric glial cells from the submucosa and lamina propria of the adult mouse. J Vis Exp. 2018;138:57629.
Jiang T, Harder B, Rojo de la Vega M, Wong PK, Chapman E, Zhang DD. p62 links autophagy and Nrf2 signaling. Free Radic Biol Med. 2015;88:199–204.
Barone FC, Barton ME, White RF, Legos JJ, Kikkawa H, Shimamura M, et al. Inhibition of phosphodiesterase type 4 decreases stress-induced defecation in rats and mice. Pharmacology. 2008;81:11–17.
Yuan F, Ren H, Tan W, Wang Y, Luo H. Effect of phosphodiesterase‐4 inhibitor rolipram on colonic hypermotility in water avoidance stress rat model. Neurogastroenterol Motil. 2022;34:e14317.
Bianchi L, Del Duca E, Romanelli M, Saraceno R, Chimenti S, Chiricozzi A. Pharmacodynamic assessment of apremilast for the treatment of moderate-to-severe plaque psoriasis. Expert Opin Drug Metab Toxicol. 2016;12:1121–8.
Holland AM, Bon-Frauches AC, Keszthelyi D, Melotte V, Boesmans W. The enteric nervous system in gastrointestinal disease etiology. Cell Mol Life Sci. 2021;78:4713–33.
Neunlist M, Rolli-Derkinderen M, Latorre R, Van Landeghem L, Coron E, Derkinderen P, et al. Enteric glial cells: recent developments and future directions. Gastroenterology. 2014;147:1230–7.
Wang P, Du C, Chen FX, Li CQ, Yu YB, Han T, et al. BDNF contributes to IBS-like colonic hypersensitivity via activating the enteroglia-nerve unit. Sci Rep. 2016;6:20320.
Sun Y, Liu X, Wang L, Li L, Quan X, Shi H, et al. Losartan attenuates acetic acid enema-induced visceral hypersensitivity by inhibiting the ACE1/Ang II/AT1 receptor axis in enteric glial cells. Eur J Pharmacol. 2023;946:175650.
Lu X, Zhang S. How tongxie-yaofang regulates intestinal synaptic plasticity by activating enteric glial cells and NGF/TrkA pathway in diarrhea-predominant irritable bowel syndrome rats. Drug Des Devel Ther. 2023;17:2969–83.
Xu S, Qin B, Shi A, Zhao J, Guo X, Dong L. Oxytocin inhibited stress induced visceral hypersensitivity, enteric glial cells activation, and release of proinflammatory cytokines in maternal separated rats. Eur J Pharmacol. 2018;818:578–84.
Hanani M, Reichenbach A. Morphology of horseradish peroxidase (HRP)-injected glial cells in the myenteric plexus of the guinea-pig. Cell Tissue Res. 1994;278:153–60.
Boesmans W, Lasrado R, Vanden Berghe P, Pachnis V. Heterogeneity and phenotypic plasticity of glial cells in the mammalian enteric nervous system. Glia. 2015;63:229–41.
Boesmans W, Nash A, Tasnády KR, Yang W, Stamp LA, Hao MM. Development, diversity, and neurogenic capacity of enteric glia. Front Cell Dev Biol. 2021;9:775102.
Seguella L, Gulbransen BD. Enteric glial biology, intercellular signalling and roles in gastrointestinal disease. Nat Rev Gastroenterol Hepatol. 2021;18:571–87.
Coelho A, Oliveira R, Antunes-Lopes T, Cruz CD. Partners in crime: NGF and BDNF in visceral dysfunction. Curr Neuropharmacol. 2019;17:1021–38.
Gross KJ, Pothoulakis C. Role of neuropeptides in inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:918–32.
Xu XJ, Zhang YL, Liu L, Pan L, Yao SK. Increased expression of nerve growth factor correlates with visceral hypersensitivity and impaired gut barrier function in diarrhoea-predominant irritable bowel syndrome: a preliminary explorative study. Aliment Pharmacol Ther. 2017;45:100–14.
Liu C, Yang J. Enteric glial cells in immunological disorders of the gut. Front Cell Neurosci. 2022;16:895871.
Diaz de Barboza G, Guizzardi S, Moine L, Tolosa de Talamoni N. Oxidative stress, antioxidants and intestinal calcium absorption. World J Gastroenterol. 2017;23:2841–53.
Buendia I, Michalska P, Navarro E, Gameiro I, Egea J, León R. Nrf2–ARE pathway: An emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol Ther. 2016;157:84–104.
Geertsema S, Bourgonje AR, Fagundes RR, Gacesa R, Weersma RK, van Goor H, et al. The NRF2/Keap1 pathway as a therapeutic target in inflammatory bowel disease. Trends Mol Med. 2023;29:830–42.
He F, Ru X, Wen T. NRF2, a transcription factor for stress response and beyond. Int J Mol Sci. 2020;21:4744.
Acknowledgements
This work was granted by National Natural Science Foundation of China (82173822) and Strategic Priority Research Program of the Chinese Academy of Sciences (XDB1060000). We thank that Prof Hai-yan Zhang for her kind assistance to this study.
Author information
Authors and Affiliations
Contributions
YHL, SYL, TY, and WT designed project and contributed to the conception. YHL, SYL, YSX, HLW, and CLF performed research. YHL, SYL, and TY analyzed data. YHL, SYL, and WT wrote the paper. All authors contributed to the collection and interpretation of data and approved the final draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, Yh., Lei, Sy., Yang, T. et al. PDE4 inhibitor apremilast ameliorates TNBS-induced irritable bowel syndrome in mice by activating the Nrf-2 signaling pathway in enteric glial cells. Acta Pharmacol Sin 47, 135–147 (2026). https://doi.org/10.1038/s41401-025-01649-w
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-025-01649-w