Abstract
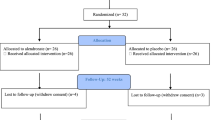
To date, monthly oral bisphosphonates have not been available in China. In this randomized, double blind, positive-controlled, multicenter phase III clinical trial, we compared the efficacy and safety of monthly minodronate versus weekly alendronate in the treatment of Chinese postmenopausal women with osteoporosis. A total of 548 participants were screened across 31 study centers, of which 330 participants were randomized into two groups: the experimental group (n = 165) received oral minodronate (50 mg/tablet once every four weeks) and alendronate placebo (once weekly), while the positive control group (n = 165) received oral alendronate (70 mg/tablet once weekly) and minodronate placebo (once every four weeks) for a duration of 48 weeks. The bone mineral density (BMD) of the lumbar spine, femoral neck and total hip were measured using dual-energy X-ray absorptiometry (DXA) at baseline and at 24 and 48 weeks. At the end of treatments, the experimental group exhibited a mean increase (SD) in BMD above the baseline at the lumbar spine, femoral neck and total hip of 4.61% (4.613%), 3.04% (4.034%) and 3.40% (3.569%), respectively, compared with those of 4.55% (3.753%), 1.86% (3.592%) and 2.30% (4.838%) in the control group. All improvements from the baseline in the two groups were statistically significant. The monthly minodronate did not cause new safety risks compared with alendronate. This study demonstrates that monthly minodronate administration is non-inferior to weekly alendronate in terms of therapeutic efficacy, while maintaining a comparable safety profile. Furthermore, the monthly dosing schedule of minodronate may significantly enhance medication adherence among osteoporosis patients, potentially improving long-term treatment outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Wang L, Yu W, Yin X, Cui L, Tang S, Jiang N, et al. Prevalence of osteoporosis and fracture in China: The China osteoporosis prevalence study. JAMA Netw Open. 2021;4:e2121106.
Zhang C, Feng J, Wang S, Gao P, Xu L, Zhu J, et al. Incidence of and trends in hip fracture among adults in urban China: a nationwide retrospective cohort study. PLoS Med. 2020;17:e1003180.
Zhang H, Gu JM, Chao AJ, Cheng Q, Teng DH, Yu JM, et al. A phase III randomized, double-blind, placebo-controlled trial of the denosumab biosimilar QL1206 in postmenopausal Chinese women with osteoporosis and high fracture risk. Acta Pharmacol Sin. 2023;44:446–53.
Gu JM, Wang L, Lin H, Chen DC, Tang H, Jin XL, et al. The efficacy and safety of weekly 35-mg risedronate dosing regimen for Chinese postmenopausal women with osteoporosis or osteopenia: 1-year data. Acta Pharmacol Sin. 2015;36:841–6.
Fleisch H. Development of bisphosphonates. Breast Cancer Res. 2002;4:30–4.
Weycker D, Lamerato L, Schooley S, Macarios D, Siu Woodworth T, Yurgin N, et al. Adherence with bisphosphonate therapy and change in bone mineral density among women with osteoporosis or osteopenia in clinical practice. Osteoporos Int. 2013;24:1483–9.
Belhassen M, Cortet B, Confavreux CB, Lamezec L, Ginoux M, Van Ganse E. Impact of bisphosphonate compliance on the risk of osteoporotic fracture in France. Arch Osteoporos. 2018;13:113.
Tosteson AN, Grove MR, Hammond CS, Moncur MM, Ray GT, Hebert GM, et al. Early discontinuation of treatment for osteoporosis. Am J Med. 2003;115:209–16.
Siris ES, Selby PL, Saag KG, Borgström F, Herings RM, Silverman SL. Impact of osteoporosis treatment adherence on fracture rates in North America and Europe. Am J Med. 2009;122:S3–13.
Dunford JE, Thompson K, Coxon FP, Luckman SP, Hahn FM, Poulter CD, et al. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther. 2001;296:235–42.
Tanishima S, Kishimoto Y, Fukata S, Mizumura H, Hagino H, Teshima R. Minodronic acid influences receptor activator of nuclear factor kappaB ligand expression and suppresses bone resorption by osteoclasts in rats with collagen-induced arthritis. Mod Rheumatol. 2007;17:198–205.
Camacho PM, Petak SM, Binkley N, Diab DL, Eldeiry LS, Farooki A, et al. American association of clinical endocrinologists/American college of endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr Pr. 2020;26:1–46.
Matsumoto T, Hagino H, Shiraki M, Fukunaga M, Nakano T, Takaoka K, et al. Effect of daily oral minodronate on vertebral fractures in Japanese postmenopausal women with established osteoporosis: a randomized placebo-controlled double-blind study. Osteoporos Int. 2009;20:1429–37.
Hagino H, Shiraki M, Fukunaga M, Nakano T, Takaoka K, Ohashi Y, et al. Three years of treatment with minodronate in patients with postmenopausal osteoporosis. J Bone Min Metab. 2012;30:439–46.
Peng C, Tian R, Li L, Zhu YK, Li SY, Ye SD, et al. A randomized double-blinded placebo-controlled clinical trial of minodronate tablet in postmenopausal Chinese women with osteoporosis. Zhonghua Fu Chan Ke Za Zhi. 2022;57:346–51.
Okazaki R, Hagino H, Ito M, Sone T, Nakamura T, Mizunuma H, et al. Efficacy and safety of monthly oral minodronate in patients with involutional osteoporosis. Osteoporos Int. 2012;23:1737–45.
Ohishi T, Fujita T, Suzuki D, Nishida T, Okabayashi R, Yamamoto K, et al. Initiation of monthly minodronate therapy at an early stage after hip fracture. J Clin Densitom. 2016;19:352–8.
Hagino H, Nishizawa Y, Sone T, Morii H, Taketani Y, Nakamura T, et al. A double-blinded head-to-head trial of minodronate and alendronate in women with postmenopausal osteoporosis. Bone. 2009;44:1078–84.
Ohishi T, Fujita T, Suzuki D, Nishida T, Yamamoto K, Okabayashi R, et al. Changes of bone mineral density and serum pentosidine during a 27-month follow-up of monthly minodronate in osteoporotic patients. Endocr Res. 2017;42:232–40.
Ito M, Sone T, Fukunaga M. Effect of minodronic acid hydrate on hip geometry in Japanese women with postmenopausal osteoporosis. J Bone Min Metab. 2010;28:334–41.
Kamimura M, Nakamura Y, Ikegami S, Komatsu M, Uchiyama S, Kato H. Monthly minodronate inhibits bone resorption to a greater extent than does monthly risedronate. Osteoporos Sarcopenia. 2016;2:170–74.
Sakai A, Ikeda S, Okimoto N, Matsumoto H, Teshima K, Okazaki Y, et al. Clinical efficacy and treatment persistence of monthly minodronate for osteoporotic patients unsatisfied with, and shifted from, daily or weekly bisphosphonates: the BP-MUSASHI study. Osteoporos Int. 2014;25:2245–53.
Ohta H, Uemura Y, Sone T, Tanaka S, Soen S, Mori S, et al. Effect of bone resorption inhibitors on serum cholesterol level and fracture risk in osteoporosis: randomized comparative study between minodronic acid and raloxifene. Calcif Tissue Int. 2023;112:430–39.
Taguchi A, Uemura Y, Imai T, Tanaka S, Ohta H, Nakamura T, et al. Incidence of osteonecrosis of the jaw in Japanese osteoporosis patients taking minodronic acid. J Bone Min Metab. 2019;37:886–92.
Hadji P, Minne H, Pfeifer M, Bourgeois P, Fardellone P, Licata A, et al. Treatment preference for monthly oral ibandronate and weekly oral alendronate in women with postmenopausal osteoporosis: A randomized, crossover study (BALTO II). Joint Bone Spine. 2008;75:303–10.
Kastelan D, Lozo P, Stamenkovic D, Miskic B, Vlak T, Kolak Z, et al. Preference for weekly and monthly bisphosphonates among patients with postmenopausal osteoporosis: results from the Croatian PROMO Study. Clin Rheumatol. 2009;28:321–6.
Iwamoto J, Okano H, Furuya T, Urano T, Hasegawa M, Hirabayashi H, et al. Patient preference for monthly bisphosphonate versus weekly bisphosphonate in a cluster-randomized, open-label, crossover trial: minodroate alendronate/risedronate trial in osteoporosis (MARTO). J Bone Min Metab. 2016;34:201–8.
Kishimoto H, Maehara M. Compliance and persistence with daily, weekly, and monthly bisphosphonates for osteoporosis in Japan: analysis of data from the CISA. Arch Osteoporos. 2015;10:231.
Acknowledgements
We thank all the patients and their investigators and their teams who participated in the present trial.
Author information
Authors and Affiliations
Contributions
ZLZ and XC take responsibility for the data, review and edit the manuscript. ZLZ, XC, YWZ, ZFC, XJW, JC, QFL, XHX, LZL, XLM, LJF, XPC, LQY, FJX, LY, JLC, DH, FH, LM, GXJ, GFY, YJB, GJX, YY, JX, YZ, WZ, YD, GPC, HMZ, CYL and JXZ conceived and designed research; ZLZ, HZ, YNH, YWZ, ZFC, XJW, JC, QFL, XHX, LZL, XLM, LJF, XPC, LQY, FJX, LY, JLC, DH, FH, LM, GXJ, GFY, YJB, GJX, YY, JX, YZ, WZ, YD, GPC, HMZ and CYL collected data and conducted research; ZLZ, HZ, YNH, YWZ, ZFC, XJW, JC, QFL, XHX, LZL, XLM, LJF, XPC, LQY, FJX, LY, JXZ, ZMZ and BJZ analyzed and interpreted data; HZ, YNH, ZLZ and XC wrote the original draft of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
JXZ, ZMZ and BJZ are employees of the study sponsor (Shandong New Time Pharmaceutical Co., Ltd, Linyi, China) at the time of study. The other authors declare that they have no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, H., Huo, Yn., Zhang, Yw. et al. A phase III clinical trial of monthly minodronate in the treatment of Chinese postmenopausal women with osteoporosis. Acta Pharmacol Sin 47, 383–390 (2026). https://doi.org/10.1038/s41401-025-01661-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-025-01661-0