Abstract
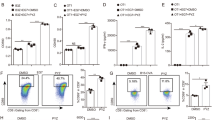
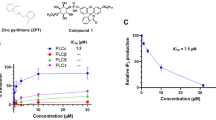
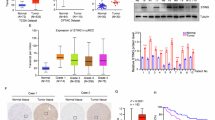
The stimulator of interferon genes (STING) is a crucial pattern recognition receptor that activates innate immunity, particularly in response to pathogen infection and various stimuli. Notably, activation of STING exhibits remarkable potential in enhancing anti-tumor immunity, underscoring the significance of discovering STING small molecule agonists. Recently, zinc pyrithione (ZPT), a marketed antifungal small molecule, has been reported to possess anti-tumor activity through various mechanisms. Our preliminary screening of STING agonists revealed that ZPT could significantly induce STING activation. In this study, we investigated whether ZPT exerted anticancer effects as a small molecule activator of STING. We showed that ZPT bound to the STING protein in vitro with KD value of 2.72 μM, and ZPT (1–16 μM) dose-dependently activated the STING-TBK1-IRF3 signaling axis in THP-1 cells. In MC38 tumor-bearing wild-type C57BL/6 mice with normal immune systems, administration of ZPT (5, 10, or 20 mg/kg, i.p., every two days for 14 days) dose-dependently inhibited the tumor growth, activated CD45+, CD3+, and CD8+ T cells in both tumors and spleens, and significantly elevated IL-6 secretion in the peripheral blood. These results highlight the potential of ZPT as an immunotherapeutic agent targeting STING.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Xiang W, Lv H, Xing F, Sun X, Ma Y, Wu L, et al. Inhibition of ACLY overcomes cancer immunotherapy resistance via polyunsaturated fatty acids peroxidation and cGAS-STING activation. Sci Adv. 2023;9:eadi2465.
Tian H, Li W, Wang G, Tian Y, Yan J, Yu X, et al. Metal-phenolic nanomaterial with organelle-level precision primes antitumor immunity via mtDNA-dependent cGAS-STING activation. Angew Chem Int Ed Engl. 2024;63:e202411498.
Li Y, Liu B, Zheng Y, Hu M, Liu LY, Li CR, et al. Photoinduction of ferroptosis and cGAS-STING activation by a H2S-responsive iridium(III) complex for cancer-specific therapy. J Med Chem. 2024;67:16235–47.
Li W, Lu L, Lu J, Wang X, Yang C, Jin J, et al. cGAS-STING-mediated DNA sensing maintains CD8+ T cell stemness and promotes antitumor T cell therapy. Sci Transl Med. 2020;12:eaay9013.
Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–91.
Hajishengallis G, Li X, Mitroulis I, Chavakis T. Trained innate immunity and its implications for mucosal immunity and inflammation. Adv Exp Med Biol. 2019;1197:11–26.
Chen K, Lai C, Su Y, Bao WD, Yang LN, Xu PP, et al. cGAS-STING-mediated IFN-I response in host defense and neuroinflammatory diseases. Curr Neuropharmacol. 2022;20:362–71.
Zhong B, Yang Y, Li S, Wang YY, Li Y, Diao F, et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29:538–50.
Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–8.
Chen Q, Sun L, Chen ZJ. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol. 2016;17:1142–9.
Erttmann SF, Swacha P, Aung KM, Brindefalk B, Jiang H, Hartlova A, et al. The gut microbiota prime systemic antiviral immunity via the cGAS-STING-IFN-I axis. Immunity. 2022;55:847–861 e10.
Cohen D, Melamed S, Millman A, Shulman G, Oppenheimer-Shaanan Y, Kacen A, et al. Cyclic GMP-AMP signalling protects bacteria against viral infection. Nature. 2019;574:691–5.
Li F, Wen Z, Wu C, Yang Z, Wang Z, Diao W, et al. Simultaneous activation of immunogenic cell death and cGAS-STING pathway by liver- and mitochondria-targeted Gold(I) complexes for chemoimmunotherapy of hepatocellular carcinoma. J Med Chem. 2024;67:1982–2003.
Jneid B, Bochnakian A, Hoffmann C, Delisle F, Djacoto E, Sirven P, et al. Selective STING stimulation in dendritic cells primes antitumor T cell responses. Sci Immunol. 2023;8:eabn6612.
Zheng W, Liu A, Xia N, Chen N, Meurens F, Zhu J. How the innate immune DNA sensing cGAS-STING pathway is involved in apoptosis. Int J Mol Sci. 2023;24:3029.
Wang X, Hu R, Song Z, Zhao H, Pan Z, Feng Y, et al. Sorafenib combined with STAT3 knockdown triggers ER stress-induced HCC apoptosis and cGAS-STING-mediated anti-tumor immunity. Cancer Lett. 2022;547:215880.
Wang J, Li S, Wang M, Wang X, Chen S, Sun Z, et al. STING licensing of type I dendritic cells potentiates antitumor immunity. Sci Immunol. 2024;9:eadj3945.
Liu J, Zhang Y, Yang B, Jia Y, Liu RT, Ding L, et al. Synergistic glutathione depletion and STING activation to potentiate dendritic cell maturation and cancer vaccine efficacy. Angew Chem Int Ed Engl. 2024;63:e202318530.
Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41:830–42.
Nicolai CJ, Wolf N, Chang IC, Kirn G, Marcus A, Ndubaku CO, et al. NK cells mediate clearance of CD8+ T cell-resistant tumors in response to STING agonists. Sci Immunol. 2020;5:eaaz2738.
Klarquist J, Hennies CM, Lehn MA, Reboulet RA, Feau S, Janssen EM. STING-mediated DNA sensing promotes antitumor and autoimmune responses to dying cells. J Immunol. 2014;193:6124–34.
Conlon J, Burdette DL, Sharma S, Bhat N, Thompson M, Jiang Z, et al. Mouse, but not human STING, binds and signals in response to the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid. J Immunol. 2013;190:5216–25.
Zhao M, Fan W, Wang Y, Qiang P, Zheng Z, Shan H, et al. M335, a novel small-molecule STING agonist activates the immune response and exerts antitumor effects. Eur J Med Chem. 2024;264:116018.
Ramanjulu JM, Pesiridis GS, Yang J, Concha N, Singhaus R, Zhang SY, et al. Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature. 2018;564:439–43.
Pan BS, Perera SA, Piesvaux JA, Presland JP, Schroeder GK, Cumming JN, et al. An orally available non-nucleotide STING agonist with antitumor activity. Science. 2020;369:eaax5164.
Chin EN, Yu C, Vartabedian VF, Jia Y, Kumar M, Gamo AM, et al. Antitumor activity of a systemic STING-activating non-nucleotide cGAMP mimetic. Science. 2020;369:993–9.
Turley PA, Fenn RJ, Ritter JC, Callow ME. Pyrithiones as antifoulants: environmental fate and loss of toxicity. Biofouling. 2005;21:31–40.
Chandler CJ, Segel IH. Mechanism of the antimicrobial action of pyrithione: effects on membrane transport, ATP levels, and protein synthesis. Antimicrob Agents Chemother. 1978;14:60–68.
Van Cutsem J, Van Gerven F, Fransen J, Schrooten P, Janssen PA. The in vitro antifungal activity of ketoconazole, zinc pyrithione, and selenium sulfide against Pityrosporum and their efficacy as a shampoo in the treatment of experimental pityrosporosis in guinea pigs. J Am Acad Dermatol. 1990;22:993–8.
Mills KJ, Hu P, Henry J, Tamura M, Tiesman JP, Xu J. Dandruff/seborrhoeic dermatitis is characterized by an inflammatory genomic signature and possible immune dysfunction: transcriptional analysis of the condition and treatment effects of zinc pyrithione. Br J Dermatol. 2012;166(Suppl 2):33–40.
Bailey P, Arrowsmith C, Darling K, Dexter J, Eklund J, Lane A, et al. A double-blind randomized vehicle-controlled clinical trial investigating the effect of ZnPTO dose on the scalp vs. antidandruff efficacy and antimycotic activity. Int J Cosmet Sci. 2003;25:183–8.
Dinning AJ, Al-Adham IS, Eastwood IM, Austin P, Collier PJ. Pyrithione biocides as inhibitors of bacterial ATP synthesis. J Appl Microbiol. 1998;85:141–6.
Zhao C, Chen X, Yang C, Zang D, Lan X, Liao S, et al. Repurposing an antidandruff agent to treating cancer: zinc pyrithione inhibits tumor growth via targeting proteasome-associated deubiquitinases. Oncotarget. 2017;8:13942–56.
Tailler M, Senovilla L, Lainey E, Thepot S, Metivier D, Sebert M, et al. Antineoplastic activity of ouabain and pyrithione zinc in acute myeloid leukemia. Oncogene. 2012;31:3536–46.
Srivastava G, Matta A, Fu G, Somasundaram RT, Datti A, Walfish PG, et al. Anticancer activity of pyrithione zinc in oral cancer cells identified in small molecule screens and xenograft model: Implications for oral cancer therapy. Mol Oncol. 2015;9:1720–35.
Zhao Y, Wang H, Duah PA, Retyunskiy V, Liu Y, Chen G. Zinc pyrithione (ZPT)-induced embryonic toxicogenomic responses reveal involvement of oxidative damage, apoptosis, endoplasmic reticulum (ER) stress and autophagy. Aquat Toxicol. 2022;248:106195.
Zhou X, Meng F, Xu B, Ma R, Cheng Y, Wu J, et al. STING promotes invasion and migration of uveal melanoma through p38‑MAPK signaling. Oncol Rep. 2024;51:23.
Lin W, Szabo C, Liu T, Tao H, Wu X, Wu J. STING trafficking activates MAPK-CREB signaling to trigger regulatory T cell differentiation. Proc Natl Acad Sci USA. 2024;121:e2320709121.
Cai D, Liu H, Wang J, Hou Y, Pang T, Lin H, et al. Balasubramide derivative 3C attenuates atherosclerosis in apolipoprotein E-deficient mice: role of AMPK-STAT1-STING signaling pathway. Aging (Albany NY). 2021;13:12160–78.
Liu X, Huang X, Luo J, Gao SN, Bai C, Xie D, et al. Low-dose radiation promotes high-fat diet-induced atherosclerosis by activating cGAS signal pathway. Biochim Biophys Acta Mol Basis Dis. 2024;1870:167443.
Pimkova Polidarova M, Brehova P, Dejmek M, Birkus G, Brazdova A. STING agonist-mediated cytokine secretion is accompanied by monocyte apoptosis. ACS Infect Dis. 2022;8:463–71.
Willemsen J, Neuhoff MT, Hoyler T, Noir E, Tessier C, Sarret S, et al. TNF leads to mtDNA release and cGAS/STING-dependent interferon responses that support inflammatory arthritis. Cell Rep. 2021;37:109977.
Zhang L, Wei X, Wang Z, Liu P, Hou Y, Xu Y, et al. NF-kappaB activation enhances STING signaling by altering microtubule-mediated STING trafficking. Cell Rep. 2023;42:112185.
McCoy CE, Carpenter S, Palsson-McDermott EM, Gearing LJ, O’Neill LA. Glucocorticoids inhibit IRF3 phosphorylation in response to Toll-like receptor-3 and -4 by targeting TBK1 activation. J Biol Chem. 2008;283:14277–85.
Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–8.
Chau TL, Goktuna SI, Rammal A, Casanova T, Duong HQ, Gatot JS, et al. A role for APPL1 in TLR3/4-dependent TBK1 and IKKepsilon activation in macrophages. J Immunol. 2015;194:3970–83.
Wu B, Li D, Bai H, Mo R, Li H, Xie J, et al. Mammalian reovirus micro1 protein attenuates RIG-I and MDA5-mediated signaling transduction by blocking IRF3 phosphorylation and nuclear translocation. Mol Immunol. 2024;170:131–43.
Lui WY, Bharti A, Wong NM, Jangra S, Botelho MG, Yuen KS, et al. Suppression of cGAS- and RIG-I-mediated innate immune signaling by Epstein-Barr virus deubiquitinase BPLF1. PLoS Pathog. 2023;19:e1011186.
Zhao B, Du F, Xu P, Shu C, Sankaran B, Bell SL, et al. A conserved PLPLRT/SD motif of STING mediates the recruitment and activation of TBK1. Nature. 2019;569:718–22.
Xuan C, Hu R. Chemical biology perspectives on STING agonists as tumor immunotherapy. ChemMedChem. 2023;18:e202300405.
Vornholz L, Isay SE, Kurgyis Z, Strobl DC, Loll P, Mosa MH, et al. Synthetic enforcement of STING signaling in cancer cells appropriates the immune microenvironment for checkpoint inhibitor therapy. Sci Adv. 2023;9:eadd8564.
Mondal I, Das O, Sun R, Gao J, Yu B, Diaz A, et al. PP2Ac deficiency enhances tumor immunogenicity by activating STING-type I interferon signaling in glioblastoma. Cancer Res. 2023;83:2527–42.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (32470793, 22271317), Guangdong Basic and Applied Basic Research Foundation (2025A1515011707), and the Shenzhen Medical Research Fund (SMRF) D2403008.
Author information
Authors and Affiliations
Contributions
MZ, WZF, LH, and ML designed the research; MZ, ZYJ, and WZF performed the experiments; MZ and WZF analyzed the data; WZF and PFQ curated the data; ZYJ and PFQ developed the methodology; ZHZ, LH, and ML contributed to the conceptualization; MZ, ZYJ, WZF, PFQ, ZHZ, and ML wrote the paper; GFL, LH, and ML supervised the research; GFL, LH, and ML acquired the funding.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, M., Jin, Zy., Fan, Wz. et al. Zinc pyrithione functions as a small-molecule STING agonist to exert antitumor immunotherapy effects. Acta Pharmacol Sin (2025). https://doi.org/10.1038/s41401-025-01674-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41401-025-01674-9