Abstract
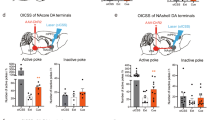
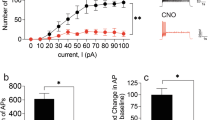
Addictive substances transform environmental cues into potent conditioned cues through reward-based associative learning. While visual cues are known to amplify drug-seeking behavior and trigger relapse, the neural circuits mediating their motivational salience remain incompletely understood. Here, we identified the superior colliculus (SC) as a critical encoder of drug-related visual information via gating reinstatement through a defined SC–VTA–NAcore pathway. We established a methamphetamine (MA) self-administration model in mice with fiber photometry, optogenetic, and chemogenetic techniques. Using fiber photometry, we discovered that the monosynaptic SC–VTA pathway exhibited selective activation during exposure to drug-paired visual cues, which demonstrated a stable cue encoding. Optogenetic inhibition of SC–VTA projections completely abolished cue-induced reinstatement, while activation potentiated reinstatement. Transsynaptic tracing confirmed a SCGlu+–VTADA+–NAcore circuit. Bidirectional manipulation of this pathway demonstrated its necessity and sufficiency for controlling cue-triggered reinstatement. Our results establish the SC as a sensory-motivational hub that transforms visual drug cues into relapse-promoting signals through a hardwired midbrain circuit. The discovery of this SC–VTA–NAcore pathway provides both a mechanistic framework for understanding cue-driven addiction and concrete targets for interventions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data available on request from the authors.
References
Deng J, Lin X, Zheng Y, Su S, Liu X, Yuan K, et al. Manipulating critical memory periods to treat psychiatry disorders. Sci Bull. 2023;68:2477–86.
Lüscher C, Robbins TW, Everitt BJ. The transition to compulsion in addiction. Nat Rev Neurosci. 2020;21:247–63.
Zhang L, Meng S, Huang E, Di T, Ding Z, Huang S, et al. High frequency deep brain stimulation of the dorsal raphe nucleus prevents methamphetamine priming-induced reinstatement of drug seeking in rats. Transl Psychiatry. 2024;14:190.
Lei J, Zhang P, Li T, Cui C, Li M, Yang X, et al. Alternating bilateral sensory stimulation alleviates alcohol-induced conditioned place preference via a superior colliculus-VTA circuit. Cell Rep. 2024;43:114383.
Morel C. Supplementary motor area cortical neuron membrane properties: key determinant for cocaine-seeking behaviors. Biol Psychiatry. 2023;94:e37–9.
Gallivan JP, Culham JC. Neural coding within human brain areas involved in actions. Curr Opin Neurobiol. 2015;33:141–9.
Zhaoping L. Understanding vision: theory, models, and data. Perception. 2016;45:1207–8.
Hafed ZM, Hoffmann KP, Chen CY, Bogadhi AR. Visual functions of the primate superior colliculus. Annu Rev Vis Sci. 2023;9:361–83.
Felsen G, Mainen ZF. Neural substrates of sensory-guided locomotor decisions in the rat superior colliculus. Neuron. 2008;60:137–48.
Baruchin LJ, Alleman M, Schroder S. Reward modulates visual responses in the superficial superior colliculus of mice. J Neurosci. 2023;43:8663–80.
Basso MA, May PJ. Circuits for action and cognition: a view from the superior colliculus. Annu Rev Vis Sci. 2017;3:197–226.
Johnson KO, Harel L, Triplett JW. Postsynaptic NMDA receptor expression is required for visual corticocollicular projection refinement in the mouse superior colliculus. J Neurosci. 2023;43:1310–20.
Ikeda T, Hikosaka O. Positive and negative modulation of motor response in primate superior colliculus by reward expectation. J Neurophysiol. 2007;98:3163–70.
Ikeda T, Hikosaka O. Reward-dependent gain and bias of visual responses in primate superior colliculus. Neuron. 2003;39:693–700.
Liu Y, Zhou ZX, Lv Q, Huang G, Zhang H, Wang YQ, et al. A superior colliculus-originating circuit prevents cocaine reinstatement via VR-based eye movement desensitization treatment. Natl Sci Rev. 2025;12:nwae467.
Willmore L, Cameron C, Yang J, Witten IB, Falkner AL. Behavioural and dopaminergic signatures of resilience. Nature. 2022;611:124–32.
Elum JE, Szelenyi ER, Juarez B, Murry AD, Loginov G, Zamorano CA, et al. Distinct dynamics and intrinsic properties in ventral tegmental area populations mediate reward association and motivation. Cell Rep. 2024;43:114668.
Stelly CE, Girven KS, Lefner MJ, Fonzi KM, Wanat MJ. Dopamine release and its control over early Pavlovian learning differs between the NAc core and medial NAc shell. Neuropsychopharmacology. 2021;46:1780–7.
Bobadilla A-C, Dereschewitz E, Vaccaro L, Heinsbroek JA, Scofield MD, Kalivas PW. Cocaine and sucrose rewards recruit different seeking ensembles in the nucleus accumbens core. Mol Psychiatry. 2020;25:3150–63.
He Y, Wang J, Li K-L, Wang YQ, Freyberg Z, Dong Y. Membrane excitability of nucleus accumbens neurons gates the incubation of cocaine craving. Neuropsychopharmacology. 2023;48:1318–27.
Liu Y, Montgomery SE, Juarez B, Morel C, Zhang S, Kong Y, et al. Different adaptations of dopamine release in Nucleus Accumbens shell and core of individual alcohol drinking groups of mice. Neuropharmacology. 2020;175:108176.
Manyi J, Xiaoyan D, Xiao H, Tai-Yun Z, Minmin L, Ning W, et al. Activation of mesocorticolimbic dopamine projections initiates cue-induced reinstatement of reward seeking in mice. Acta Pharmacol Sin. 2022;43:2276–88.
He Y, Huang YH, Schlüter OM, Dong Y. Cue- versus reward-encoding basolateral amygdala projections to nucleus accumbens. eLife. 2023;12:e89766.
Fassier C, Nicol X. Retinal axon interplay for binocular mapping. Front Neural Circuits. 2021;15:679440.
Tien NW, Vitale C, Badea TC, Kerschensteiner D. Layer-specific developmentally precise axon targeting of transient suppressed-by-contrast retinal ganglion cells. J Neurosci. 2022;42:7213–21.
Li PY, Jing MY, Cun XF, Wu N, Li J, Song R. The neural circuit of Superior colliculus to ventral tegmental area modulates visual cue associated with rewarding behavior in optical intracranial self-stimulation in mice. Neurosci Lett. 2024;842:137997.
Pradel K, Tymorek A, Marzec M, Chrobok Ł, Solecki W, Błasiak T. Superior colliculus controls the activity of the substantia nigra pars compacta and ventral tegmental area in an asymmetrical manner. J Neurosci. 2025;45:e1976222024.
Awathale SN, Waghade AM, Kawade HM, Jadhav G, Choudhary AG, Sagarkar S, et al. Neuroplastic changes in the superior colliculus and hippocampus in self-rewarding paradigm: importance of visual cues. Mol Neurobiol. 2022;59:890–915.
Igelstrom KM, Herbison AE, Hyland BI. Enhanced c-Fos expression in superior colliculus, paraventricular thalamus and septum during learning of cue-reward association. Neuroscience. 2010;168:706–14.
Engel L, Wolff AR, Blake M, Collins VL, Sinha S, Saunders BT. Dopamine neurons drive spatiotemporally heterogeneous striatal dopamine signals during learning. Curr Biol. 2024;34:3086–101.e4.
Ferguson LM, Ahrens AM, Longyear LG, Aldridge JW. Neurons of the ventral tegmental area encode individual differences in motivational “wanting” for reward cues. J Neurosci. 2020;40:8951–63.
Xu W, Wang M, Yang G, Mo F, Liu Y, Shan J, et al. Neuronal activity in the ventral tegmental area during goal-directed navigation recorded by low-curvature microelectrode arrays. Microsyst Nanoeng. 2024;10:145.
Collins AL, Aitken TJ, Greenfield VY, Ostlund SB, Wassum KM. Nucleus accumbens acetylcholine receptors modulate dopamine and motivation. Neuropsychopharmacology. 2016;41:2830–8.
Dai B, Sun F, Tong X, Ding Y, Kuang A, Osakada T, et al. Responses and functions of dopamine in nucleus accumbens core during social behaviors. Cell Rep. 2022;40:111246.
Goedhoop JN, van den Boom BJG, Robke R, Veen F, Fellinger L, van Elzelingen W, et al. Nucleus accumbens dopamine tracks aversive stimulus duration and prediction but not value or prediction error. eLife. 2022;11:e82711.
Solie C, Contestabile A, Espinosa P, Musardo S, Bariselli S, Huber C, et al. Superior colliculus to VTA pathway controls orienting response and influences social interaction in mice. Nat Commun. 2022;13:817.
Desarkar P, Vicario CM, Soltanlou M. Non-invasive brain stimulation in research and therapy. Sci Rep. 2024;14:29334.
Bharath MM, Paliwal VK, Batra S, Mishra P, Mishra N, Saini R. Repetitive transcranial magnetic stimulation in new daily persistent headache patients: a single arm open label study. J Headache Pain. 2024;25:155.
Birreci D, De Riggi M, Costa D, Angelini L, Cannavacciuolo A, Passaretti M, et al. The role of non-invasive brain modulation in identifying disease biomarkers for diagnostic and therapeutic purposes in Parkinsonism. Brain Sci. 2024;14:695.
Song P, Li S, Shao Y, Zhu S, Wang Y, Xu P, et al. High frequency-rTMS of the left DLPFC relieve headaches and enhance frontal-temporal connectivity in migraine. Clin Neurophysiol. 2025;173:166–72.
Cox SS, Connolly DJ, Peng X, Badran BW. A comprehensive review of low-intensity focused ultrasound parameters and applications in neurologic and psychiatric disorders. Neuromodulation. 2025;28:1–15.
Zhong YX, Liao JC, Liu X, Tian H, Deng LR, Long L. Low intensity focused ultrasound: a new prospect for the treatment of Parkinson’s disease. Ann Med. 2023;55:2251145.
Acknowledgements
This study was supported by the National Key Research and Development Program of China (2023YFC3304201).
Author information
Authors and Affiliations
Contributions
XFC: investigation, methodology, data curation. MYJ: writing—original draft, validation. MDY: visualization, methodology, data curation. NW: writing—review and editing, investigation, conceptualization. JL: writing—review and editing, supervision, investigation, conceptualization. RS: writing—review and editing, supervision, investigation, funding acquisition, conceptualization.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cun, Xf., Jing, My., Yang, Md. et al. A novel superior colliculus circuit mediates visual cue-driven methamphetamine taking and seeking. Acta Pharmacol Sin (2026). https://doi.org/10.1038/s41401-025-01733-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41401-025-01733-1