Abstract
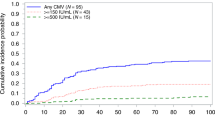
We compared CMV outcomes of three prophylactic approaches used for CBT and haploidentical cord transplants from December 2009 through 2018: letermovir (n = 32) through day 100 post transplant, “valacyclovir day 100” (valacyclovir 2 g orally three times daily through day 100) (n = 60), and “valacyclovir hospital discharge” (valacyclovir 2 g orally three times daily through hospital discharge then acyclovir 800 mg twice daily) (n = 41). Through day 100, none in the letermovir group, six (10%) in the “valacyclovir day 100,” and nine (22%) in the “valacyclovir hospital discharge” group required CMV directed treatment (p = 0.005 and 0.06 comparing letermovir to “valacyclovir hospital discharge” and “valacyclovir day 100”). Fewer patients in the letermovir group (n = 7, 22%) had any CMV reactivation versus the “valacyclovir day 100” group (n = 20, 33%) versus the “valacyclovir hospital discharge” group (n = 23, 57%) (p = 0.003 and 0.21 comparing letermovir to “valacyclovir hospital discharge” and “valacyclovir day 100”). Among patients not reactivating CMV before 100 days, reactivation rates between day 100 and 180 were higher in the letermovir and “valacyclovir day 100” groups than the “valacyclovir hospital discharge” group. Letermovir is safe and effective compared with alternative prophylaxis approaches following CBT through day 100. Reactivation and monitoring after day 100 remain potential concerns.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Milano F, Pergam SA, Xie H, Leisenring WM, Gutman JA, Riffkin I, et al. Intensive strategy to prevent CMV disease in seropositive umbilical cord blood transplant recipients. Blood. 2011;118:5689–96.
Dahi PB, Perales MA, Devlin SM, Olson A, Lubin M, Gonzales AM, et al. Incidence, nature and mortality of cytomegalovirus infection after double-unit cord blood transplant. Leuk Lymphoma. 2015;56:1799–805.
Takami A, Mochizuki K, Asakura H, Yamazaki H, Okumura H, Nakao S. High incidence of cytomegalovirus reactivation in adult recipients of an unrelated cord blood transplant. Haematol-Hematol J. 2005;90:1290–2.
Beck JC, Wagner JE, DeFor TE, Brunstein CG, Schleiss MR, Young JA, et al. Impact of cytomegalovirus (CMV) reactivation after umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2010;16:215–22.
Lau C, Politikos I, Maloy M, Naputo K, Afuye A, Devlin SM, et al. Letermovir prophylaxis demonstrates high efficacy in adult cytomegalovirus (CMV) seropositive cord blood transplant (CBT) recipients: a comparison with pre-letermovir era CBT controls. Biol Blood Marrow Transplant. 2019;25:S94–95.
Marty FM, Ljungman P, Chemaly RF, Maertens J, Dadwal SS, Duarte RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med. 2017;377:2433–44.
Hill JA, Mayer BT, Xie H, Leisenring WM, Huang ML, Stevens-Ayers T, et al. The cumulative burden of double-stranded DNA virus detection after allogeneic HCT is associated with increased mortality. Blood. 2017;129:2316–25.
Ballen K, Woo Ahn K, Chen M, Abdel-Azim H, Ahmed I, Aljurf M, et al. Infection rates among acute leukemia patients receiving alternative donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22:1636–45.
Brown JA, Boussiotis VA. Umbilical cord blood transplantation: basic biology and clinical challenges to immune reconstitution. Clin Immunol. 2008;127:286–97.
Komanduri KV, St John LS, de Lima M, McMannis J, Rosinski S, McNiece I, et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood. 2007;110:4543–51.
Ljungman P, de La Camara R, Milpied N, Volin L, Russell CA, Crisp A, et al. Randomized study of valacyclovir as prophylaxis against cytomegalovirus reactivation in recipients of allogeneic bone marrow transplants. Blood. 2002;99:3050–6.
Winston DJ, Yeager AM, Chandrasekar PH, Snydman DR, Petersen FB, Territo MC, et al. Randomized comparison of oral valacyclovir and intravenous ganciclovir for prevention of cytomegalovirus disease after allogeneic bone marrow transplantation. Clin Infect Dis. 2003;36:749–58.
Hill JA, Pergam SA, Cox E, Xie H, Leisenring WM, Boeckh M, et al. A modified intensive strategy to prevent cytomegalovirus disease in seropositive umbilical cord blood transplantation recipients. Biol Blood Marrow Transplant. 2018;24:2094–2100.
Knoll BM, Peixoto D, Koo S, Hammond SP, Ho VT, Antin JH, et al. Cytomegalovirus infection among cord blood allogeneic transplantation recipients: low incidence of cytomegalovirus events without high-dose valacyclovir prophylaxis. Biol Blood Marrow Transplant. 2018;24:2164–5.
Liu H, van Besien K. Alternative donor transplantation—“mixing and matching”: the role of combined cord blood and haplo-identical donor transplantation (haplo-cord SCT) as a treatment strategy for patients lacking standard donors? Curr Hematol Malig Rep. 2015;10:1–7.
Kwon M, Bautista G, Balsalobre P, Sanchez-Ortega I, Serrano D, Anguita J, et al. Haplo-cord transplantation using CD34+ cells from a third-party donor to speed engraftment in high-risk patients with hematologic disorders. Biol Blood Marrow Transplant. 2014;20:2015–22.
Ljungman P, Boeckh M, Hirsch HH, Josephson F, Lundgren J, Nichols G, et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis. 2017;64:87–91.
Kroll JL, Beam C, Li S, Viscidi R, Dighero B, Cho A, et al. Reactivation of latent viruses in individuals receiving rituximab for new onset type 1 diabetes. J Clin Virol. 2013;57:115–9.
Jain N, Liu H, Artz AS, Anastasi J, Odenike O, Godley LA, et al. Immune reconstitution after combined haploidentical and umbilical cord blood transplant. Leuk Lymphoma. 2013;54:1242–9.
Kwon M, Balsalobre P, Serrano D, Perez Corral A, Buno I, Anguita J, et al. Single cord blood combined with HLA-mismatched third party donor cells: comparable results to matched unrelated donor transplantation in high-risk patients with hematologic disorders. Biol Blood Marrow Transplant. 2013;19:143–9.
Liu H, Rich ES, Godley L, Odenike O, Joseph L, Marino S, et al. Reduced-intensity conditioning with combined haploidentical and cord blood transplantation results in rapid engraftment, low GVHD, and durable remissions. Blood. 2011;118:6438–45.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sharma, P., Gakhar, N., MacDonald, J. et al. Letermovir prophylaxis through day 100 post transplant is safe and effective compared with alternative CMV prophylaxis strategies following adult cord blood and haploidentical cord blood transplantation. Bone Marrow Transplant 55, 780–786 (2020). https://doi.org/10.1038/s41409-019-0730-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41409-019-0730-y
This article is cited by
-
Outcomes of allogeneic hematopoietic cell transplantation under letermovir prophylaxis for cytomegalovirus infection
Annals of Hematology (2024)
-
Real-World Safety and Effectiveness of Letermovir in Patients Undergoing Allogenic Hematopoietic Stem Cell Transplantation: Final Results of Post-Marketing Surveillance in Japan
Clinical Drug Investigation (2024)
-
Dynamics of polyclonal immuno-reconstitution after allogeneic transplant with post-transplant cyclophosphamide and letermovir
Bone Marrow Transplantation (2023)
-
Monitoring for virus-specific T-cell responses and viremia in allogeneic HSCT recipients: a survey from the EBMT Cellular Therapy & Immunobiology Working Party
Bone Marrow Transplantation (2023)
-
Risk factors for late cytomegalovirus infection after completing letermovir prophylaxis
International Journal of Hematology (2022)