Abstract
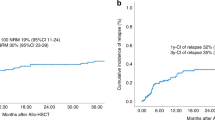
Primary immunodeficiencies (PID) are heterogeneous inborn errors of the immune system. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is curative and safe at the pediatric age but remains underperformed in adults. We report our experience on 32 consecutive adult patients with various PID including 17 (53%) with a combined immune deficiency, six (19%) with a disease of immune dysregulation and nine (28%) with a chronic granulomatous disease (CGD) who underwent an allo-HSCT between 2011 and 2020. The median age at transplant was 27 years (17–41). All assessable patients engrafted. The majority of patients received a fludarabine-Busulfan (FB) based regimen (FB2-3 in 16, FB4 in 12). Overall survival (OS) was 80.4% (100% for CGD and 74% for other PID patients) at 9 months and beyond (median follow-up 51.6 months). Six patients died, all in the first-year post-transplant. Cumulative incidences of grade II–IV acute GVHD/chronic GVHD were 18%/22%. Stem cell source, GVHD prophylaxis and conditioning intensity had no impact on OS. All surviving patients had over 90% donor chimerism, immune reconstitution, no sign of active PID related complications and were clinically improved. Allo-HSCT is effective in young adults PID patients with an acceptable toxicity and should be discussed in case of life-threatening PID.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others

Data availability
Correspondence and requests for materials should be addressed to Ambroise Marçais or Felipe Suarez.
References
Tangye SG, Al-Herz W, Bousfiha A, Chatila T, Cunningham-Rundles C, Etzioni A, et al. Human inborn errors of immunity: 2019 update on the classification from the international union of immunological societies expert committee. J Clin Immunol. 2020;40:24–64.
Bousfiha A, Jeddane L, Picard C, Al-Herz W, Ailal F, Chatila T, et al. Human inborn errors of immunity: 2019 update of the IUIS phenotypical classification. J Clin Immunol. 2020;40:66–81.
McCusker C, Upton J, Warrington R. Primary immunodeficiency. Allergy Asthma Clin Immunol Off J Can Soc Allergy Clin Immunol 2018; 14. https://doi.org/10.1186/s13223-018-0290-5.
Mitchell R. Hematopoietic stem cell transplantation beyond severe combined immunodeficiency: seeking a cure for primary immunodeficiency. J Allergy Clin Immunol Pr. 2019;7:776–85.
Fischer A, Landais P, Friedrich W, Morgan G, Gerritsen B, Fasth A, et al. European experience of bone-marrow transplantation for severe combined immunodeficiency. Lancet Lond Engl. 1990;336:850–4.
Pai S-Y, Logan BR, Griffith LM, Buckley RH, Parrott RE, Dvorak CC, et al. Transplantation outcomes for severe combined immunodeficiency, 2000-2009. N. Engl J Med. 2014;371:434–46.
Ferrua F, Galimberti S, Courteille V, Slatter MA, Booth C, Moshous D, et al. Hematopoietic stem cell transplantation for CD40 ligand deficiency: results from an EBMT/ESID-IEWP-SCETIDE-PIDTC study. J Allergy Clin Immunol. 2019;143:2238–53.
Fischer A, Landais P, Friedrich W, Gerritsen B, Fasth A, Porta F, et al. Bone marrow transplantation (BMT) in Europe for primary immunodeficiencies other than severe combined immunodeficiency: a report from the European Group for BMT and the European Group for Immunodeficiency. Blood. 1994;83:1149–54.
Antoine C, Müller S, Cant A, Cavazzana-Calvo M, Veys P, Vossen J, et al. Long-term survival and transplantation of haemopoietic stem cells for immunodeficiencies: report of the European experience 1968-99. Lancet Lond Engl. 2003;361:553–60.
Moratto D, Giliani S, Bonfim C, Mazzolari E, Fischer A, Ochs HD, et al. Long-term outcome and lineage-specific chimerism in 194 patients with Wiskott-Aldrich syndrome treated by hematopoietic cell transplantation in the period 1980-2009: an international collaborative study. Blood. 2011;118:1675–84.
Morillo-Gutierrez B, Beier R, Rao K, Burroughs L, Schulz A, Ewins A-M, et al. Treosulfan-based conditioning for allogeneic HSCT in children with chronic granulomatous disease: a multicenter experience. Blood. 2016;128:440–8.
Güngör T, Teira P, Slatter M, Stussi G, Stepensky P, Moshous D, et al. Reduced-intensity conditioning and HLA-matched haemopoietic stem-cell transplantation in patients with chronic granulomatous disease: a prospective multicentre study. Lancet. 2014;383:436–48.
Castagnoli R, Delmonte OM, Calzoni E, Notarangelo LD. Hematopoietic stem cell transplantation in primary immunodeficiency diseases: current status and future perspectives. Front Pediatr. 2019;7:295.
Myers LA, Patel DD, Puck JM, Buckley RH. Hematopoietic stem cell transplantation for severe combined immunodeficiency in the neonatal period leads to superior thymic output and improved survival. Blood. 2002;99:872–8.
Morris EC, Albert MH. Allogeneic HSCT in adolescents and young adults with primary immunodeficiencies. Front Pediatr. 2019;7:437.
Barlogis V, Mahlaoui N, Auquier P, Pellier I, Fouyssac F, Vercasson C, et al. Physical health conditions and quality of life in adults with primary immunodeficiency diagnosed during childhood: A French Reference Center for PIDs (CEREDIH) study. J Allergy Clin Immunol. 2017;139:1275–1281.e7.
Barlogis V, Mahlaoui N, Auquier P, Fouyssac F, Pellier I, Vercasson C, et al. Burden of poor health conditions and quality of life in 656 children with primary immunodeficiency. J Pediatr. 2018;194:211–217.e5.
Chiesa R, Wang J, Blok H-J, Hazelaar S, Neven B, Moshous D, et al. Hematopoietic cell transplantation in chronic granulomatous disease: a study of 712 children and adults. Blood. 2020;136:1201–11.
Fox TA, Chakraverty R, Burns S, Carpenter B, Thomson K, Lowe D, et al. Successful outcome following allogeneic hematopoietic stem cell transplantation in adults with primary immunodeficiency. Blood. 2018;131:917–31.
Wehr C, Gennery AR, Lindemans C, Schulz A, Hoenig M, Marks R, et al. Multicenter experience in hematopoietic stem cell transplantation for serious complications of common variable immunodeficiency. J Allergy Clin Immunol. 2015;135:988–997.e6.
Albert MH, Hauck F, Wiebking V, Aydin S, Notheis G, Koletzko S, et al. Allogeneic stem cell transplantation in adolescents and young adults with primary immunodeficiencies. J Allergy Clin Immunol Pr. 2018;6:298–301.e2.
Alizadeh M, Bernard M, Danic B, Dauriac C, Birebent B, Lapart C, et al. Quantitative assessment of hematopoietic chimerism after bone marrow transplantation by real-time quantitative polymerase chain reaction. Blood. 2002;99:4618–25.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2009;15:1628–33.
Le Bourgeois A, Labopin M, Blaise D, Ceballos P, Vigouroux S, Peffault de Latour R, et al. Reduced-intensity versus reduced-toxicity myeloablative fludarabine/busulfan-based conditioning regimens for allografted non-Hodgkin lymphoma adult patients: a retrospective study on behalf of the Société Francophone de Greffe de Moelle et de Thérapie Cellulaire. Ann Oncol J Eur Soc Med Oncol. 2017;28:2191–8.
Kharfan-Dabaja MA, Labopin M, Bazarbachi A, Hamladji RM, Blaise D, Socié G, et al. Comparing i.v. BU dose intensity between two regimens (FB2 vs FB4) for allogeneic HCT for AML in CR1: a report from the Acute Leukemia Working Party of EBMT. Bone Marrow Transpl. 2014;49:1170–5.
Marsh RA, Rao MB, Gefen A, Bellman D, Mehta PA, Khandelwal P, et al. Experience with Alemtuzumab, Fludarabine, and Melphalan reduced-intensity conditioning hematopoietic cell transplantation in patients with nonmalignant diseases reveals good outcomes and that the risk of mixed chimerism depends on underlying disease, stem cell source, and Alemtuzumab regimen. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2015;21:1460–70.
Neven B, Diana J-S, Castelle M, Magnani A, Rosain J, Touzot F, et al. Haploidentical hematopoietic stem cell transplantation with post-transplant cyclophosphamide for primary immunodeficiencies and inherited disorders in children. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2019;25:1363–73.
Thakar MS, Broglie L, Logan B, Artz A, Bunin N, Burroughs LM, et al. The Hematopoietic Cell Transplant Comorbidity Index predicts survival after allogeneic transplant for nonmalignant diseases. Blood. 2019;133:754–62.
Nishimura A, Aoki Y, Ishiwata Y, Ichimura T, Ueyama J, Kawahara Y et al. Hematopoietic cell transplantation with reduced intensity conditioning using fludarabine/busulfan or fludarabine/melphalan for primary immunodeficiency diseases. J Clin Immunol 2021. https://doi.org/10.1007/s10875-021-00966-z.
Peric Z, Labopin M, Peczynski C, Polge E, Cornelissen J, Carpenter B, et al. Comparison of reduced-intensity conditioning regimens in patients with acute lymphoblastic leukemia >45 years undergoing allogeneic stem cell transplantation-a retrospective study by the Acute Leukemia Working Party of EBMT. Bone Marrow Transpl. 2020;55:1560–9.
Author information
Authors and Affiliations
Contributions
AM and FS designed and supervised the study and wrote the manuscript. AM, NM, BN, FL, EC, HS, MC, LJC, OL, CP, DM, OH and AF provided clinical care for the patients included in the study. MJ performed post-vaccination serological assays. VA performed chimerism analysis. PvE performed HLA typing and donor selection and CP performed genetic analysis and immunological cellular reconstitution.
Corresponding authors
Ethics declarations
COMPETING INTERESTS
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Marçais, A., Mahlaoui, N., Neven, B. et al. Curative allogeneic hematopoietic stem cell transplantation following reduced toxicity conditioning in adults with primary immunodeficiency. Bone Marrow Transplant 57, 1520–1530 (2022). https://doi.org/10.1038/s41409-022-01739-x
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41409-022-01739-x