Abstract
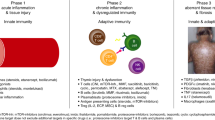
Chronic graft-versus-host disease (cGVHD) is a serious complication of allogeneic hematopoietic cell transplant. The development of cGVHD involves a complex, multistep process that is characterized by early inflammation and tissue injury, followed by chronic inflammation, aberrant tissue repair, and fibrosis. Systemic corticosteroids remain the first line of treatment for cGVHD. New treatments for patients with cGVHD for whom treatment has failed or who develop steroid-dependent cGVHD are now available; these include ibrutinib, ruxolitinib, and belumosudil. Treatment selection may be based on the patient’s individual needs, graft-versus-host disease organ involvement, and comorbidities. However, as therapeutic options for patients without a treatment response or with only a partial response remain an unmet need, new agents are under investigation. Furthermore, patients with cGVHD can develop multiorgan involvement and frequently require specialized care. A multidisciplinary team approach that focuses on the individual’s needs and quality of life is strongly encouraged.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Zeiser R, Blazar BR. Pathophysiology of chronic graft-versus-host disease and therapeutic targets. N Engl J Med. 2017;377:2565–79.
Ciurea SO, Zhang MJ, Bacigalupo AA, Bashey A, Appelbaum FR, Aljitawi OS, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126:1033–40.
Ratanatharathorn V, Nash RA, Przepiorka D, Devine SM, Klein JL, Weisdorf D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92:2303–14.
Kröger N, Solano C, Wolschke C, Bandini G, Patriarca F, Pini M, et al. Antilymphocyte globulin for prevention of chronic graft-versus-host disease. N Engl J Med. 2016;374:43–53.
Bachier CR, Aggarwal SK, Hennegan K, Milgroom A, Francis K, Dehipawala S, et al. Epidemiology and treatment of chronic graft-versus-host disease post-allogeneic hematopoietic cell transplantation: a US claims analysis. Transpl Cell Ther. 2021;27:504.
Choi SW, Levine JE, Ferrara JL. Pathogenesis and management of graft-versus-host disease. Immunol Allergy Clin North Am. 2010;30:75–101.
Kumar S, Mohammadpour H, Cao X. Targeting cytokines in GVHD therapy. J Immunol Res Ther. 2017;2:90–9.
Wu T, Young JS, Johnston H, Ni X, Deng R, Racine J, et al. Thymic damage, impaired negative selection, and development of chronic graft-versus-host disease caused by donor CD4+ and CD8+ T cells. J Immunol. 2013;191:488–99.
Whitehouse G, Gray E, Mastoridis S, Merritt E, Kodela E, Yang JH, et al. IL-2 therapy restores regulatory T-cell dysfunction induced by calcineurin inhibitors. Proc Natl Acad Sci USA. 2017;114:7083–8.
Leveque-El Mouttie L, Koyama M, Le Texier L, Markey KA, Cheong M, Kuns RD, et al. Corruption of dendritic cell antigen presentation during acute GVHD leads to regulatory T-cell failure and chronic GVHD. Blood. 2016;128:794–804.
Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4(+) regulatory T cell function. J Exp Med. 2002;196:851–7.
Sarantopoulos S, Stevenson KE, Kim HT, Bhuiya NS, Cutler CS, Soiffer RJ, et al. High levels of B-cell activating factor in patients with active chronic graft-versus-host disease. Clin Cancer Res. 2007;13:6107–14.
Allen JL, Tata PV, Fore MS, Wooten J, Rudra S, Deal AM, et al. Increased BCR responsiveness in B cells from patients with chronic GVHD. Blood. 2014;123:2108–15.
MacDonald KPA, Hill GR, Blazar BR. Chronic graft-versus-host disease: biological insights from preclinical and clinical studies. Blood. 2017;129:13–21.
Rick K, Saskia E, Anton H. B-cell involvement in chronic graft-versus-host disease. Haematologica. 2008;93:1702–11.
Kuzmina Z, Gounden V, Curtis L, Avila D, Rnp TT, Baruffaldi J, et al. Clinical significance of autoantibodies in a large cohort of patients with chronic graft-versus-host disease defined by NIH criteria. Am J Hematol. 2015;90:114–9.
Schutt SD, Fu J, Nguyen H, Bastian D, Heinrichs J, Wu Y, et al. Inhibition of BTK and ITK with ibrutinib is effective in the prevention of chronic graft-versus-host disease in mice. PLoS One. 2015;10:e0137641.
Svegliati S, Olivieri A, Campelli N, Luchetti M, Poloni A, Trappolini S, et al. Stimulatory autoantibodies to PDGF receptor in patients with extensive chronic graft-versus-host disease. Blood. 2007;110:237–41.
Forcade E, Kim HT, Cutler C, Wang K, Alho AC, Nikiforow S, et al. Circulating T follicular helper cells with increased function during chronic graft-versus-host disease. Blood. 2016;127:2489–97.
Hong YQ, Wan B, Li XF. Macrophage regulation of graft-vs-host disease. World J Clin Cases. 2020;8:1793–805.
Hill GR, Olver SD, Kuns RD, Varelias A, Raffelt NC, Don AL, et al. Stem cell mobilization with G-CSF induces type 17 differentiation and promotes scleroderma. Blood. 2010;116:819–28.
Brüggen MC, Klein I, Greinix H, Bauer W, Kuzmina Z, Rabitsch W, et al. Diverse T-cell responses characterize the different manifestations of cutaneous graft-versus-host disease. Blood. 2014;123:290–9.
Alexander KA, Flynn R, Lineburg KE, Kuns RD, Teal BE, Olver SD, et al. CSF-1-dependant donor-derived macrophages mediate chronic graft-versus-host disease. J Clin Invest. 2014;124:4266–80.
Forcade E, Paz K, Flynn R, Griesenauer B, Amet T, Li W, et al. An activated Th17-prone T cell subset involved in chronic graft-versus-host disease sensitive to pharmacological inhibition. JCI Insight. 2017;2:e92111.
Dander E, Balduzzi A, Zappa G, Lucchini G, Perseghin P, Andrè V, et al. Interleukin-17-producing T-helper cells as new potential player mediating graft-versus-host disease in patients undergoing allogeneic stem-cell transplantation. Transplantation. 2009;88:1261–72.
Weiss JM, Chen W, Nyuydzefe MS, Trzeciak A, Flynn R, Tonra JR, et al. ROCK2 signaling is required to induce a subset of T follicular helper cells through opposing effects on STATs in autoimmune settings. Sci Signal. 2016;9:ra73.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transpl. 2005;11:945–56.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transpl. 2015;21:389–401.
Cuvelier GDE, Schoettler M, Buxbaum NP, Pinal-Fernandez I, Schmalzing M, Distler JHW, et al. Toward a better understanding of the atypical features of chronic graft-versus-host disease: a report from the 2020 National Institutes of Health Consensus Project Task Force. Transpl Cell Ther. 2022;28:426–45.
Lee SJ. Classification systems for chronic graft-versus-host disease. Blood. 2017;129:30–7.
Dignan FL, Amrolia P, Clark A, Cornish J, Jackson G, Mahendra P, et al. Diagnosis and management of chronic graft-versus-host disease. Br J Haematol. 2012;158:46–61.
Wolff D, Gerbitz A, Ayuk F, Kiani A, Hildebrandt GC, Vogelsang GB, et al. Consensus conference on clinical practice in chronic graft-versus-host disease (GVHD): first-line and topical treatment of chronic GVHD. Biol Blood Marrow Transpl. 2010;16:1611–28.
National Comprehensive Cancer Network. NCCN Guidelines for Hematopoietic Cell Transplantation. Version 1. 2023. https://www.nccn.org/guidelines/guidelines-process/transparency-process-and-recommendations/GetFileFromFileManagerGuid?FileManagerGuidId=1f518816-3ec1-4a1a-92f2-635ba824581e. Accessed January 18, 2024.
Flowers ME, Martin PJ. How we treat chronic graft-versus-host disease. Blood. 2015;125:606–15.
Wolff D, Fatobene G, Rocha V, Kröger N, Flowers ME. Steroid-refractory chronic graft-versus-host disease: treatment options and patient management. Bone Marrow Transpl. 2021;56:2079–87.
Schoemans HM, Lee SJ, Ferrara JL, Wolff D, Levine JE, Schultz KR, et al. EBMT-NIH-CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transpl. 2018;53:1401–15.
Janssen Pharmaceuticals. Imbruvica (ibrutinib) [package insert]. U.S Food and Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/217003s000lbl.pdf.
Jaglowski SM, Blazar BR. How ibrutinib, a B-cell malignancy drug, became an FDA-approved second-line therapy for steroid-resistant chronic GVHD. Blood Adv. 2018;2:2012–9.
Miklos D, Cutler CS, Arora M, Waller EK, Jagasia M, Pusic I, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood. 2017;130:2243–50.
Waller EK, Miklos D, Cutler C, Arora M, Jagasia MH, Pusic I, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy: 1-year update of a phase 1b/2 study. Biol Blood Marrow Transpl. 2019;25:2002–7.
Chin K-K, Kim HT, Inyang E-A, Ho V, Koreth J, Romee R, et al. Ibrutinib in steroid-refractory chronic graft-versus-host disease, a single-center experience. Transpl Cell Ther. 2021;27:990.
Jakafi (ruxolitinib). Package insert. Incyte Corporation; 2023.
Zeiser R, Blazar BR. Acute graft-versus-host disease - biologic process, prevention, and therapy. N Engl J Med. 2017;377:2167–79.
Zeiser R, Polverelli N, Ram R, Hashmi SK, Chakraverty R, Middeke JM, et al. Ruxolitinib for glucocorticoid-refractory chronic graft-versus-host disease. N Engl J Med. 2021;385:228–38.
Redondo S, Esquirol A, Novelli S, Caballero AC, Garrido A, Oñate G, et al. Efficacy and safety of ruxolitinib in steroid-refractory/dependent chronic graft-versus-host disease: real-world data and challenges. Transpl Cell Ther. 2022;28:43.
Ferreira AM, Szor RS, Molla VC, Seiwald MC, de Moraes PA, da Fonseca ARBM, et al. Long-term follow-up of ruxolitinib in the treatment of steroid-refractory chronic graft-versus-host disease. Transpl Cell Ther. 2021;27:777.
Wu H, Shi J, Luo Y, Tan Y, Zhang M, Lai X, et al. Evaluation of ruxolitinib for steroid-refractory chronic graft-vs-host disease after allogeneic hematopoietic stem cell transplantation. JAMA Netw Open. 2021;4:e2034750–e2034750.
Sanofi Pharmaceuticals. Rezurock (belumosudil) [package insert]. U.S Food and Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214783s000lbl.pdf.
Zanin-Zhorov A, Weiss JM, Nyuydzefe MS, Chen W, Scher JU, Mo R, et al. Selective oral ROCK2 inhibitor down-regulates IL-21 and IL-17 secretion in human T cells via STAT3-dependent mechanism. Proc Natl Acad Sci USA. 2014;111:16814–9.
Rozo C, Chinenov Y, Maharaj RK, Gupta S, Leuenberger L, Kirou KA, et al. Targeting the RhoA-ROCK pathway to reverse T-cell dysfunction in SLE. Ann Rheum Dis. 2017;76:740–7.
Cutler C, Lee SJ, Arai S, Rotta M, Zoghi B, Lazaryan A, et al. Belumosudil for chronic graft-versus-host disease after 2 or more prior lines of therapy: the ROCKstar Study. Blood. 2021;138:2278–89.
Holtzman NG, Im A, Ostojic A, Curtis LM, Parsons-Wandell L, Nashed J, et al. Efficacy and safety of baricitinib in refractory chronic graft-versus-host disease (cGVHD): preliminary analysis results of a phase 1/2 study. Blood. 2020;136:1.
Koshy AG, Kim HT, Liegel J, Arnason J, Ho VT, Antin JH, et al. Phase 2 clinical trial evaluating abatacept in patients with steroid-refractory chronic graft-versus-host disease. Blood. 2023;141:2932–43.
Pidala J, Bhatt VR, Hamilton B, Pusic I, Wood WA, Onstad L, et al. Ixazomib for treatment of refractory chronic graft-versus-host disease: a Chronic GVHD Consortium phase II trial. Biol Blood Marrow Transpl. 2020;26:1612–9.
Study of baricitinib, a JAK1/ 2 inhibitor, in chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. https://classic.clinicaltrials.gov/ct2/show/NCT02759731. Accessed May 16, 2024.
Kitko CL, Arora M, DeFilipp Z, Zaid MA, Stasi AD, Radojcic V, et al. Axatilimab for chronic graft-versus-host disease after failure of at least two prior systemic therapies: results of a phase I/II study. J Clin Oncol. 2023;41:1864–75.
Wolf D, Cutler C, Lee SJ, Pusic I, Bittencourt H, White J, et al. Safety and efficacy of axatilimab at 3 different doses in patients with chronic graft-versus-host disease (AGAVE-201). Blood. 2023;142:1.
Visintini C, Mansutti I, Palese A. Medication adherence among allogeneic haematopoietic stem cell transplant recipients: a systematic review. Cancers. 2023;15:2452.
Couriel D, Carpenter PA, Cutler C, Bolaños-Meade J, Treister NS, Gea-Banacloche J, et al. Ancillary Therapy and Supportive Care of Chronic Graft-versus-Host Disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: V. Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transpl. 2006;12:375–96.
Gresch B, Kirsch M, Fierz K, Halter JP, Nair G, Denhaerynck K, et al. Medication nonadherence to immunosuppressants after adult allogeneic haematopoietic stem cell transplantation: a multicentre cross-sectional study. Bone Marrow Transpl. 2017;52:304–6.
Verbrugghe M, Verhaeghe S, Lauwaert K, Beeckman D, Van Hecke A. Determinants and associated factors influencing medication adherence and persistence to oral anticancer drugs: A systematic review. Cancer Treat Rev. 2013;39:610–21.
Saleem MS, Aljurf M, Srivastava A, Shamsi T, Lu PH, Hamidieh AA, et al. Challenges in managing graft-versus-host disease in developing countries: a perspective. Bone Marrow Transpl. 2019;54:641–7.
Morrison CF, Martsolf DM, Wehrkamp N, Tehan R, Pai ALH. Medication adherence in hematopoietic stem cell transplantation: a review of the literature. Biol Blood Marrow Transpl. 2017;23:562–8.
Chieng R, Coutsouvelis J, Poole S, Dooley MJ, Booth D. Implementation of a clinical pharmacy service to an allogeneic stem cell transplant ambulatory clinic. J Pharm Pr Res. 2014;44:105–7.
Wenzel F, Pralong A, Holtick U, Scheid C, Herling M, Simon ST. Burden and needs of patients with severe GvHD from the supportive and palliative care perspective-a literature review. Cancers. 2021;13:2697.
Wolff D, Bertz H, Greinix H, Lawitschka A, Halter J, Holler E. The treatment of chronic graft-versus-host disease: consensus recommendations of experts from Germany, Austria, and Switzerland. Dtsch Arztebl Int. 2011;108:732–40.
Lee SJ, Wolff D, Kitko C, Koreth J, Inamoto Y, Jagasia M, et al. Measuring therapeutic response in chronic graft-versus-host disease. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-Versus-Host Disease: IV. The 2014 Response Criteria Working Group Report. Biol Blood Marrow Transpl. 2015;21:984–99.
A study of axatilimab at 3 different doses in participants with chronic graft versus host disease (cGVHD) (AGAVE-201). https://classic.clinicaltrials.gov/ct2/show/NCT04710576. Accessed May 16, 2024.
Acknowledgements
This work was supported, in part, by the National Institutes of Health (P01 CA23766 [to Memorial Sloan Kettering Cancer Center], P30 CA008748 [to Memorial Sloan Kettering Cancer Center], and R01 HL164902 [to DMP]). Editorial support in the preparation of this manuscript was provided by David B. Sewell, MA, MFA, of the Memorial Sloan Kettering Cancer Center, Department of Surgery. Graphic support was provided by ICON PLC and funded by Incyte under the authors’ guidance.
Author information
Authors and Affiliations
Contributions
GV, SE, AP, and DMP designed the project, identified and analyzed the literature, wrote and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
SE has received speaking honorarium from Incyte. DMP has served as an advisory board member for Evive Biotechnology (Shanghai; formerly Generon [Shanghai]), as an advisory board member or consultant for Sanofi, CareDx, Ceramedix, and Incyte, and receives research funding from Incyte and Sanofi. AP is a current employee of Flatiron Health and a current equity holder in Roche. The other authors have no conflicts of interest to declare. Graphic support was provided by ICON PLC and funded by Incyte under the authors’ guidance.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vadakkel, G., Eng, S., Proli, A. et al. Updates in chronic graft-versus-host disease: novel treatments and best practices in the current era. Bone Marrow Transplant 59, 1360–1368 (2024). https://doi.org/10.1038/s41409-024-02370-8
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41409-024-02370-8
This article is cited by
-
Real-world experience of belumosudil and belumosudil/ruxolitinib combination in steroid-refractory chronic graft-versus-host disease
Bone Marrow Transplantation (2025)
-
Quantifying the Clinical and Economic Burden of Graft-Versus-Host Disease Following Allogeneic Haematopoietic Stem Cell Transplantation in England: A Retrospective Cohort Study
Advances in Therapy (2025)