Abstract
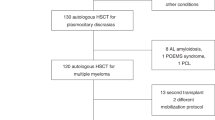
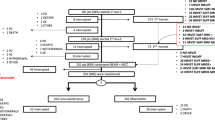
Autologous peripheral blood stem cell (PBSC) transplantation is a standard treatment of multiple myeloma (MM), Hodgkin lymphoma and various subtypes of non-Hodgkin lymphoma. Cryopreservation of hematopoietic stem cells is standard practice that allows time for delivery of conditioning regimen prior to cell infusion. The aim of this Worldwide Network for Blood & Marrow Transplantation (WBMT) work was to assess existing evidence on non-cryopreserved autologous transplants through a systematic review/meta-analysis, to study feasibility and safety of this approach. We searched PubMed, Web of Science and SCOPUS for studies that utilized non-cryopreserved autologous PBSC transplantation. Identified literature was reviewed for information on mobilization, apheresis, preservation and viability, conditioning regimen, engraftment, response, and survival. Results highlight collective experience from 19 transplant centers (1686 patients), that performed autologous transplants using non-cryopreserved PBSCs. The mean of infused CD34+ was 5.6 × 106/kg. Stem cell viability at transplantation was >90% in MM and >75% in lymphomas, after a storage time of 24–144 h at +4 °C. Mean time-to-neutrophil engraftment was 12 days and 15.3 days for platelets. Pooled proportion estimates of day 100 transplant-related mortality and graft failure were 1% and 0%, respectively. Non-cryopreservation of apheresed autologous PBSCs appears feasible and safe.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
Change history
24 June 2025
The original online version of this article was revised: In this article the author name Dietger Niederwiser has been corrected to Dietger Niederwieser and fig. 3 replaced.
30 June 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41409-025-02664-5
References
Kessinger A, Armitage JO, Landmark J, Weisenburger D. Reconstitution of human hematopoietic function with autologous cryopreserved circulating stem cells. Exp Hematol. 1986;14:192–6.
Kessinger A, Armitage JO, Landmark JD, Smith DM, Weisenburger DD. Autologous peripheral hematopoietic stem cell transplantation restores hematopoietic function following marrow ablative therapy. 1988;71:723–7.
Abdrabou AK, Sharif FA, Fakih RE, Hashmi S, Khafaga YM, Alhayli S, et al. Outcomes of autologous stem cell transplantation for multiple myeloma in Saudi Arabia. Ann Saudi Med. 2021;41:198–205.
Niederwieser D, Baldomero H, Bazuaye N, Bupp C, Chaudhri N, Corbacioglu S, et al. One and a half million hematopoietic stem cell transplants: continuous and differential improvement in worldwide access with the use of non-identical family donors. Haematologica. 2022;107:1045.
Fermand J-P, Katsahian S, Divine M, Leblond V, Dreyfus F, Macro M, et al. High-dose therapy and autologous blood stem-cell transplantation compared with conventional treatment in myeloma patients aged 55 to 65 years: long-term results of a randomized control trial from the Group Myelome-Autogreffe. J Clin Oncol. 2005;23:9227–33.
Philip T, Guglielmi C, Hagenbeek A, Somers R, Van Der Lelie H, Bron D, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333:1540–5.
André M, Henry-Amar M, Pico J-L, Brice P, Blaise D, Kuentz M, et al. Comparison of high-dose therapy and autologous stem-cell transplantation with conventional therapy for Hodgkin’s disease induction failure: a case-control study. J Clin Oncol. 1999;17:222.
Carella AM, Bellei M, Brice P, Gisselbrecht C, Visani G, Colombat P, et al. High-dose therapy and autologous stem cell transplantation versus conventional therapy for patients with advanced Hodgkin’s lymphoma responding to front-line therapy: long-term results. Haematologica. 2009;94:146.
Gorin N. Collection, manipulation and freezing of haemopoietic stem cells. Clin Haematol. 1986;15:19–48.
Billen D. Recovery of lethally irradiat-ed mice by treatment with bone marrow cells maintained in vitro. nature1957;179:574–5.
Ahmed T, Wuest D, Ciavarella D, Ayello J, Feldman EJ, Biguzzi S, et al. Marrow storage techniques: a clinical comparison of refrigeration versus cryopreservation. Acta Haematol. 1991;85:173–8.
Sierra J, Conde E, Iriondo A, Brunet S, Marin J, de Oteiza JP, et al. Frozen vs. nonfrozen bone marrow for autologous transplantation in lymphomas: a report from the Spanish GEL/TAMO Cooperative Group. Ann Hematol. 1993;67:111–4.
Preti R, Razis E, Ciavarella D, Fan Y, Kuhns R, Cook P, et al. Clinical and laboratory comparison study of refrigerated and cryopreserved bone marrow for transplantation. Bone Marrow Transplant. 1994;13:253–60.
Hechler G, Weide R, Heymanns J, Köppler H, Havemann K. Storage of noncryopreserved periphered blood stem cells for transplantation. Ann Hematol. 1996;72:303–6.
Wannesson L, Panzarella T, Mikhael J, Keating A. Feasibility and safety of autotransplants with noncryopreserved marrow or peripheral blood stem cells: a systematic review. Ann Oncol. 2007;18:623–32.
Al-Anazi KA. Autologous hematopoietic stem cell transplantation for multiple myeloma without cryopreservation. Bone Marrow Res. 2012;2012:917361.
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M. et al. The NewcastleOttawa Scale (NOS) for assessing the quality if nonrandomized studies in metaanalyses. Ontario, Canada. 2024. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–14.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135.
Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Stat. 1950;21:607–11.
Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67:974–8.
Lewis S, Clarke MJB. Forest plots: trying to see the wood and the trees. 2001;322:1479–80.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. 2003;327:557–60.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9.
Papadimitriou CA, Dimopoulos MA, Kouvelis V, Kostis E, Kapsimali V, Contoyannis D, et al. Non‐cryopreserved peripheral blood progenitor cells collected by a single very large‐volume leukapheresis: a simplified and effective procedure for support of high‐dose chemotherapy. J Clin Apher. 2000;15:236–41.
Ruiz-Argüelles GJ, Gómez-Rangel D, Ruiz-Delgado GJ, Ruiz-Argüelles A, Pérez-Romano B, Rivadeneyra L. Results of an autologous noncryopreserved, unmanipulated peripheral blood hematopoietic stem cell transplant program: a single-institution, 10-year experience. Acta Haematol. 2003;110:179–83.
Cuellar-Ambrosi F, Karduss U, Gomez W, Mondragon M, Velasquez-Lopera M, Calle S, editors. Hematologic reconstitution following high-dose and supralethal chemoradiotherapy using stored, noncryopreserved autologous hematopoietic stem cells. Transplantation Proc. 2004;36:1704–5.
Mabed M, Shamaa S. High-dose chemotherapy plus non-cryopreserved autologous peripheral blood stem cell transplantation rescue for patients with refractory or relapsed Hodgkin disease. Biol Blood Marrow Transpl. 2006;12:942–8.
Mabed M, Al-Kgodary T. Cyclophosphamide, etoposide and carboplatine plus non-cryopreserved autologous peripheral blood stem cell transplantation rescue for patients with refractory or relapsed non-Hodgkin’s lymphomas. Bone marrow Transplant. 2006;37:739–43.
López-Otero A, Ruiz-Delgado G, Ruiz-Argüelles G. A simplified method for stem cell autografting in multiple myeloma: a single institution experience. Bone Marrow Transplant. 2009;44:715–9.
Ramzi M, Zakerinia M, Nourani H, Dehghani M, Vojdani R, Haghighinejad H. Non‐cryopreserved hematopoietic stem cell transplantation in multiple myeloma, a single center experience. Clin Transplant. 2012;26:117–22.
Ramzi M, Mohamadian M, Vojdani R, Dehghani M, Nourani H, Zakerinia M, et al. Autologous noncryopreserved hematopoietic stem cell transplant with CEAM as a modified conditioning regimen in patients with Hodgkin lymphoma: a single-center experience with a new protocol. Exp Clin Transpl. 2012;10:163–7.
Bekadja M-A, Brahimi M, Osmani S, Arabi A, Bouhass R, Yafour N, et al. A simplified method for autologous stem cell transplantation in multiple myeloma. Hematol Oncol Stem Cell Ther. 2012;5:49–53.
Kayal S, Sharma A, Iqbal S, Tejomurtula T, Cyriac SL, Raina V. High-dose chemotherapy and autologous stem cell transplantation in multiple myeloma: a single institution experience at All India Institute of Medical Sciences, New Delhi, using non-cryopreserved peripheral blood stem cells. Clin Lymphoma Myeloma Leuk. 2014;14:140–7.
Bekadja MA, Brahimi M, Osmani S, Yafour N, Krim A, Serradj F, et al. Hematopoietic stem cell transplantation in Algeria. Hematol Oncol Stem Cell Ther. 2017;10:311–4.
Sarmiento M, Ramírez P, Parody R, Salas M, Beffermann N, Jara V, et al. Advantages of non-cryopreserved autologous hematopoietic stem cell transplantation against a cryopreserved strategy. Bone Marrow Transplant. 2018;53:960–6.
Kardduss-Urueta A, Gale RP, Gutierrez-Aguirre CH, Herrera-Rojas MA, Murrieta-Álvarez I, Perez-Fontalvo R, et al. Freezing the graft is not necessary for autotransplants for plasma cell myeloma and lymphomas. Bone Marrow Transpantl. 2018;53:457–60.
Naithani R, Dayal N, Pathak S, Rai R. Hematopoietic stem cell transplantation using non-cryopreserved peripheral blood stem cells graft is effective in multiple myeloma and lymphoma. Bone Marrow Transplant. 2018;53:1198–200.
Kulkarni U, Devasia AJ, Korula A, Fouzia N, Nisham P, Samoon YJ, et al. Use of non-cryopreserved peripheral blood stem cells is associated with adequate engraftment in patients with multiple myeloma undergoing an autologous transplant. Biol Blood Marrow Transplant. 2018;24:e31–5.
Bittencourt M, Mariano L, Moreira F, Schmidt-Filho J, Mendrone-Jr A, Rocha V. Cryopreserved versus non-cryopreserved peripheral blood stem cells for autologous transplantation after high-dose Melphalan in multiple myeloma: comparative analysis. Bone marrow Transplant. 2019;54:138–41.
Jennane S, Hasnaoui N, Mahtat E, Merimi F, Bougar S, El Maaroufi H, et al. Non-cryopreserved peripheral blood stem cells autologous transplantation in multiple myeloma: bicentric study. Transfus Clin Biol. 2020;27:152–6.
Piriyakhuntorn P, Tantiworawit A, Rattanathammethee T, Hantrakool S, Chai-Adisaksopha C, Rattarittamrong E. et al. Outcomes of non-cryopreserved versus cryopreserved peripheral blood stem cells for autologous stem cell transplantation in multiple myeloma. Ann Transplant. 2020;25:e927084-1–7.
Bekadja M-A, Boumendil A, Blaise D, Chevallier P, Peggs KS, Salles G, et al. Non-cryopreserved hematopoietic stem cells in autograft patients with lymphoma: a matched-pair analysis comparing a single center experience with the use of cryopreserved stem cells reported to the European Society for Blood and Marrow Transplantation registry. Cytotherapy. 2021;23:483–7.
Fleming K, Hubel A. Cryopreservation of hematopoietic and non-hematopoietic stem cells. Transfus Apheresis Sci. 2006;34:309–15.
Lobo F, Kessinger A, Landmark J, Smith D, Weisenburger D, Wigton R, et al. Addition of peripheral blood stem cells collected without mobilization techniques to transplanted autologous bone marrow did not hasten marrow recovery following myeloablative therapy. Bone marrow Transplant. 1991;8:389–92.
Smith R, Sweetenham J. A mononuclear cell dose of 3 x 10 (8)/kg predicts early multilineage recovery in patients with malignant lymphoma treated with carmustine, etoposide, Ara-C and melphalan (BEAM) and peripheral blood progenitor cell transplantation. Exp Hematol. 1995;23:1581–8.
Bekadja M, Talhi S, Amani K, Osmani S, Brahimi M, Mazari M, et al. Outcomes of modified-eam conditioned autologous non-cryopreserved hematopoietic sct for lymphoma. a retrospective single-centre study. Bone Marrow Transplant. 2018;53:1596–8.
Garifullin A, Voloshin S, Linnikov S, Kuzyaeva A, Balashova V, Chubukina Z. The use of non-cryopreserved and cryopreserved hematopoietic stem cells for autotransplantation in multiple myeloma. HemaSphere. 2021;5:494–5.
Lanza F, Campioni DC, Hellmann A, Milone G, Wahlin A, Walewski J, et al. Individual quality assessment of autografting by probability estimation for clinical endpoints: a prospective validation study from the European group for blood and marrow transplantation. Biol Blood Marrow Transplant. 2013;19:1670–6.
Bekadja MA, Omani S, Talhi S, Brahimi M, Yafour N, Arabi A, et al. L’autogreffe de cellules souches périphériques (CSP) non cryopréservées dans les lymphomes de Hodgkin (LH). Expérience de I’EHU 1er novembre d’Oran. Revue Algérienne d’Hématologie. 2015;10-11:40-45
Author information
Authors and Affiliations
Contributions
Study concept: MAB, MAK-D, REF, MA. Study design: MAB, MAK-D, REF, AK, TE, MA. Data collection: TE. Statistical analysis: TE. Interpretation of results: MAB, DN, MAK-D, REF, LG, IY-A, HG, DJW, SG, SOA, CC, SKH, AR, UG, AB, NH, AA, MP, AH, JS, YK, AK, TE, DMc, NW, RG, MM, YA, MK, AS, DR, MA, WR. Manuscript writing: MAB, DN, MAK-D, REF, LG, IY-A, HG, DJW, SG, SOA, CC, SKH, AR, UG, AB, NH, AA, MP, AH, JS, YK, AK, TE, DMc, NW, RG, MM, YA, MK, AS, DR, MA, WR.
Corresponding authors
Ethics declarations
Competing interests
MAB, DN, REF, DJW, SG, AR, AB, AH, YK, AK, TE, MM, WR declare no conflicts of interest MAK-D: declares research/grant from Bristol Myers Squibb, Novartis, and Pharmacyclics, and lecture/honoraria from Kite Pharma; LG: declares relationship with Bristol Myers Squibb, Sanofi, Janssen, Pfizer; IY-A: declares honoraria from Kite Pharma, Novartis and Bristol Myers Squibb; HG: declares speaker bureau and consultancy for Therakos, Gilead, Novartis, Stemline, Neovii, Sanofi, and Takeda; SOA: declares advisory board with Kite Pharma and Novartis, and speaker honoraria from Kite Pharma, Novartis, and Johnson & Johnson; CC: declares honoraria (personal and institutional) for lectures and advisory boards from Bristol Myers Squibb, Kite Pharma/Gilead, Janssen, Jazz, Novartis, and Miltenyi Biotec; SKH: declares educational/travel grants from Novartis, Pfizer, Janssen, Therakos, Vertex, MSD, Roche; UG: declares consultancy/Honoraria :Kite Pharma, Incyte, Astellas, Jazz,and Vor; NH: honoria from Janssen, Novartis, Takeda, Abbvie, Roche, Astellas, Bigene; AA: declares lecture-Advisor /honoraria from Kite Pharma, Novartis, Takeda and Janssen; MP: declares research with Bristol Myers Squibb, Janssen, Kite Pharma, Novartis, and consultancy for Bristol Myers Squibb, Novartis, and honoraria from Gilead; JS: declares consultancy for Sanofi and ADRx, honoraria from Sanofi, Alexion, AstraZeneca Rare Disease, Prevail Therapeutics (Eli–Lilly), Pfizer, Sobi Pharmaceuticals, and Novartis, advisory committees for Sanofi, AstraZeneca Rare Disease, Prevail Therapeutics (Eli–Lilly), Pfizer, Sobi Pharmaceuticals, Novartis, speaker bureau for Sanofi, AstraZeneca Rare Disease, Prevail Therapeutics (Eli–Lilly), Pfizer, Sobi Pharmaceuticals, Novartis; DMc: declares lecture/honoraria from GSK, Novartis, Abbvie. Research funding from Imago Biosciences; NW: declares speakers fees from BMS Celgene, Kite Gilead, Novartis, Pierre Fabre, Sanofi Genzyme, Therakos Mallinckrodt, Travel reimbursement from Jannsen, Pierre Fabre; RG: declares speaking honoraria from Biotest, Pfizer, Medac, Neovii and Magenta; YA: declares lecture/honoraria from Otsuka Pharmaceutical Co., Ltd, Chugai Pharmaceutical Co., Ltd., Novartis Pharma KK, Meiji Seika Pharma Co., Ltd, Janssen Pharmaceutical K.K., and consultancy fee from JCR Pharmaceuticals Co., Ltd and Kyowa Kirin Co., Ltd; MK: declares non-specified relationship with Kite Pharma, Takeda and Gilead; AS: declares honoraria from Takeda, Bristol Myers Squibb /Celgene, MSD, Janssen, Amgen, Novartis, Gilead Kite, Sanofi, Roche, Genmab, AbbVie, Jazz Pharmaceuticals, consultancy from Takeda, Bristol Myers Squibb/Celgene, Novartis, Janssen, Gilead, Sanofi, Genmab, AbbVie, speaker bureau for Takeda and Research support from Takeda; MA: declares lecture/honoraria from Kite Pharma and Vertex Pharma.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In this article the author name Dietger Niederwiser has been corrected to Dietger Niederwieser and fig. 3 replaced.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bekadja, M.A., Niederwieser, D., Kharfan-Dabaja, M.A. et al. Non-cryopreserved autologous peripheral blood stem cell transplantation for multiple myeloma and lymphoma in countries with limited resources: practice considerations from the Worldwide Network for Blood and Marrow Transplantation. Bone Marrow Transplant 60, 19–27 (2025). https://doi.org/10.1038/s41409-024-02431-y
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41409-024-02431-y