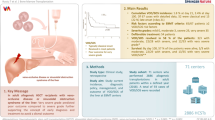
Abstract
This international questionnaire survey aimed to explore the current incidence, diagnostic policies, management, and outcomes of veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) among healthcare providers involved in the management of these patients. A questionnaire was e-mailed to practitioners with an interest in allogeneic hematopoietic cell transplantation (allo-HCT). Of the respondents, 144 of 215 (67.0%) felt that early detection or diagnosis of VOD/SOS was difficult. Regarding diagnostic criteria, 142 (66.1%) already declared using the 2023 EBMT refined criteria. Most respondents (163/215, 75.8%) found these recent refined EBMT criteria useful for diagnosis, and 193 (89.8%) found the severity criteria easy to use. The major risk factors identified for VOD/SOS were a second allo-HCT (41.4%), pre-existing liver disease (54.9%), and prior use of antibody-drug conjugates (49.8%). Preferences for starting VOD/SOS treatment varied, with 61 (28.4%) preferring initiating therapy at a mild stage, and 122 (56.7%) preferring the moderate stage. In summary, this survey provided valuable insight into the challenges and opportunities of the identification and management of VOD/SOS. By improving current knowledge and increasing collaboration among healthcare professionals, early detection, management, and clinical outcomes for patients with this potentially serious complication can also be improved.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Data sharing is available through the IACH office (info@clinical-hematology.org) and Dr Myriam Labopin (myriam.labopin@upmc.fr).
References
Vythoulkas D, Tsirigotis P, Griniezaki M, Konstantellos I, Lazana I. Endothelial dysfunction syndromes after allogeneic stem cell transplantation. Cancers (Basel). 2023;15:680.
Corbacioglu S, Jabbour EJ, Mohty M. Risk factors for development of and progression of hepatic veno-occlusive disease/sinusoidal obstruction syndrome. Biol Blood Marrow Transplant. 2019;25:1271–80.
Marcoux C, Saliba RM, Wallis W, Khazal S, Ragoonanan D, Rondon G, et al. Incidence and risk factors of early onset VOD/SOS differ in younger vs older adults after stem cell transplantation. Blood Adv. 2024;8:1128–36.
Corbacioglu S, Carreras E, Ansari M, Balduzzi A, Cesaro S, Dalle JH, et al. Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in pediatric patients: a new classification from the European society for blood and marrow transplantation. Bone Marrow Transplant. 2018;53:138–45.
Corbacioglu S, Topaloglu O, Aggarwal S. A systematic review and meta-analysis of studies of defibrotide prophylaxis for veno-occlusive disease/sinusoidal obstruction syndrome. Clin Drug Investig. 2022;42:465–76.
Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44:778–83.
Mohty M, Malard F, Alaskar AS, Aljurf M, Arat M, Bader P, et al. Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a refined classification from the European society for blood and marrow transplantation (EBMT). Bone Marrow Transplant. 2023;58:749–54.
Ichikawa H, Yakushijin K, Kurata K, Tsuji T, Takemoto N, Joyce M, et al. Utility of the refined EBMT diagnostic and severity criteria 2023 for sinusoidal obstruction syndrome/veno-occlusive disease. Bone Marrow Transplant. 2024;59:518–25.
Cairo MS, Cooke KR, Lazarus HM, Chao N. Modified diagnostic criteria, grading classification and newly elucidated pathophysiology of hepatic SOS/VOD after haematopoietic cell transplantation. Br J Haematol. 2020;190:822–36.
Dignan FL, Wynn RF, Hadzic N, Karani J, Quaglia A, Pagliuca A, et al. BCSH/BSBMT guideline: diagnosis and management of veno-occlusive disease (sinusoidal obstruction syndrome) following haematopoietic stem cell transplantation. Br J Haematol. 2013;163:444–57.
Richardson PG, Riches ML, Kernan NA, Brochstein JA, Mineishi S, Termuhlen AM, et al. Phase 3 trial of defibrotide for the treatment of severe veno-occlusive disease and multi-organ failure. Blood. 2016;127:1656–65.
Nauffal M, Kim HT, Richardson PG, Soiffer RJ, Antin JH, Cutler C, et al. Defibrotide: real-world management of veno-occlusive disease/sinusoidal obstructive syndrome after stem cell transplant. Blood Adv. 2022;6:181–8.
Bearman SI, Anderson GL, Mori M, Hinds MS, Shulman HM, McDonald GB. Venoocclusive disease of the liver: development of a model for predicting fatal outcome after marrow transplantation. J Clin Oncol. 1993;11:1729–36.
Mohty M, Malard F, Abecassis M, Aerts E, Alaskar AS, Aljurf M, et al. Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2016;51:906–12.
Bonifazi F, Barbato F, Ravaioli F, Sessa M, Defrancesco I, Arpinati M, et al. Diagnosis and treatment of VOD/SOS after allogeneic hematopoietic stem cell transplantation. Front Immunol. 2020;11:489.
Wallhult E, Kenyon M, Liptrott S, Mank A, Ní Chonghaile M, Babic A, et al. Management of veno-occlusive disease: the multidisciplinary approach to care. Eur J Haematol. 2017;98:322–9.
Author information
Authors and Affiliations
Contributions
MM and ML conceived and designed the study. All authors collected the data. MM and ML analyzed the data. MM and ML wrote the manuscript. All authors critically revised and approved the final manuscript. MM is the guarantors of the study, had full access to all the data, and took responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Competing interests
Ahmed Alaskar: Received honoraria/consultancy fees: Novartis, Abbvie, Janssen, Takeda, Kyowa Kirin, Gilead, Roche, Sanofi. Participated in company sponsored speakers bureau: Janssen, Kyowa Kirin, Sanofi. Michelle Kenyon: Received honoraria/consultancy fees: Jazz Pharmaceuticals, Sanofi, Roche, Mallinkrodt, Vertex. Speakers bureau: Jazz Pharmaceuticals, Sanofi, Pfizer.
Ethics approval
The IACH steering committee (www.iach.org) approved the conduct of this survey according to standard professional practices.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Larue, M., Labopin, M., Brissot, E. et al. An international survey to better understand the current incidence, severity, and management of VOD/SOS. Bone Marrow Transplant 60, 28–31 (2025). https://doi.org/10.1038/s41409-024-02434-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41409-024-02434-9
This article is cited by
-
Defibrotide for prophylaxis of sinusoidal obstruction syndrome/veno-occlusive disease (SOS/VOD) in pediatric high-risk patients: consensus guidelines from the European Society for Blood and Marrow Transplantation (EBMT)
Bone Marrow Transplantation (2026)
-
Management of liver sinusoidal obstruction syndrome/veno-occlusive disease in adults: a 2025 perspective from an international expert group
Bone Marrow Transplantation (2025)