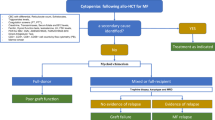
Abstract
Significant efforts have been made to effectively select myelofibrosis (MF) patients who can benefit from allogeneic hematopoietic cell transplantation (allo-HCT), the only current cure for MF. The recent EBMT/ELN 2024 recommendations offer valuable guidance for hematologists and transplant physicians. However, several grey areas remain in day-to-day clinical practice regarding the feasibility and optimal preparation for transplantation in patients with this disease. Effective spleen size reduction, often achieved with JAK inhibitors, appears crucial for transplant success. For resistant cases, switching JAK inhibitors, splenectomy, or spleen irradiation may be considered, taking into account patient profiles, treatment availability and center preferences. Managing splanchnic vein thromboses, portal, and pulmonary hypertension is critical as these conditions may affect transplant outcomes. Cytopenias, particularly transfusion-dependent anemia and thrombocytopenia, complicate treatment and impact on outcomes, though new drugs show promise. Comorbidities play a significant role and tools like the Hematopoietic Cell Transplantation-Comorbidity Index (HCT-CI) and frailty assessments are useful for evaluating transplant risks while allowing the implementation of corrective measures. Especially in low- and medium-income countries where access to novel therapies may be challenging, allo-HCT still represents an attractive therapeutic option for MF. Future directions include integrating new therapeutics into the transplant algorithm and leveraging artificial intelligence for more informed risk assessment, highlighting the need for tailored approaches to improve allo-HCT outcomes in such a setting.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka HM, et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140:1200–28. https://doi.org/10.1182/blood.2022015850.
Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36:1703–19. https://doi.org/10.1038/s41375-022-01613-1.
Polverelli N, Breccia M, Benevolo G, Martino B, Tieghi A, Latagliata R, et al. Risk factors for infections in myelofibrosis: role of disease status and treatment. a multicenter study of 507 patients. Am J Hematol. 2017;92:37–41. https://doi.org/10.1002/ajh.24572.
Tefferi A. Primary myelofibrosis: 2023 update on diagnosis, risk-stratification, and management. Am J Hematol. 2023;98:801–21. https://doi.org/10.1002/ajh.26857.
Kröger NM, Deeg JH, Olavarria E, Niederwieser D, Bacigalupo A, Barbui T, et al. Indication and management of allogeneic stem cell transplantation in primary myelofibrosis: a consensus process by an EBMT/ELN international working group. Leukemia. 2015;29:2126–33. https://doi.org/10.1038/leu.2015.233.
Kröger N, Bacigalupo A, Barbui T, Ditschkowski M, Gagelmann N, Griesshammer M. et al. Indication and management of allogeneic haematopoietic stem-cell transplantation in myelofibrosis: updated recommendations by the EBMT/ELN International Working Group. Lancet Haematol. 2024;11:e62–74. https://doi.org/10.1016/S2352-3026(23)00305-8.
Barosi G. Myelofibrosis with myeloid metaplasia: diagnostic definition and prognostic classification for clinical studies and treatment guidelines. J Clin Oncol. 1999;17:2954–2954.
Visani G, Finelli C, Castelli U, Petti MC, Ricci P, Vianelli N, et al. Myelofibrosis with myeloid metaplasia: clinical and haematological parameters predicting survival in a series of 133 patients. Br J Haematol. 1990;75:4–9.
Polverelli N, Mauff K, Kröger N, Robin M, Beelen D, Beauvais D, et al. Impact of spleen size and splenectomy on outcomes of allogeneic hematopoietic cell transplantation for myelofibrosis: a retrospective analysis by the chronic malignancies working party on behalf of European society for blood and marrow transplantation (EBMT). Am J Hematol. 2021;96:69–79.
Luther M, Henes FO, Zabelina T, Massoud R, Janson D, Wolschke C, et al. Spleen volume and length determined by computed tomography impact outcome after allogeneic stem cell transplantation for myelofibrosis. Bone Marrow Transplant. 2023;58:755–61.
Passamonti F, Mora B. Myelofibrosis. Blood 2023;141:1954–70.
Polverelli N, Hernández-Boluda JC, Czerw T, Barbui T, D’Adda M, Deeg HJ, et al. Splenomegaly in patients with primary or secondary myelofibrosis who are candidates for allogeneic hematopoietic cell transplantation: a Position Paper on behalf of the Chronic Malignancies Working Party of the EBMT. Lancet Haematol. 2023;10:e59–70.
Tremblay D, Schwartz M, Bakst R, Patel R, Schiano T, Kremyanskaya M, et al. Modern management of splenomegaly in patients with myelofibrosis. Ann Hematol. 2020;99:1441–51.
Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807.
Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–98.
Pardanani A, Tefferi A. How I treat myelofibrosis after failure of JAK inhibitors. Blood. 2018;132:492–500. https://doi.org/10.1182/blood-2018-02-785923.
Kröger N, Sbianchi G, Sirait T, Wolschke C, Beelen D, Passweg J, et al. Impact of prior JAK-inhibitor therapy with ruxolitinib on outcome after allogeneic hematopoietic stem cell transplantation for myelofibrosis: a study of the CMWP of EBMT. Leukemia 2021;35:3551–60.
Mascarenhas J, Hoffman R, Talpaz M, Gerds AT, Stein B, Gupta V, et al. Pacritinib vs best available therapy, including ruxolitinib, in patients with myelofibrosis. JAMA Oncol. 2018;4:652.
Harrison CN, Schaap N, Vannucchi AM, Kiladjian JJ, Tiu RV, Zachee P, et al. Janus kinase-2 inhibitor fedratinib in patients with myelofibrosis previously treated with ruxolitinib (JAKARTA-2): a single-arm, open-label, non-randomised, phase 2, multicentre study. Lancet Haematol. 2017;4:e317–24.
Gerds AT, Verstovsek S, Vannucchi AM, Al-Ali HK, Lavie D, Kuykendall AT, et al. Momelotinib versus danazol in symptomatic patients with anaemia and myelofibrosis previously treated with a JAK inhibitor (MOMENTUM): an updated analysis of an international, double-blind, randomised phase 3 study. Lancet Haematol. 2023;10:e735–46.
Verstovsek S, Gerds AT, Vannucchi AM, Al-Ali HK, Lavie D, Kuykendall AT, et al. Momelotinib versus danazol in symptomatic patients with anaemia and myelofibrosis (MOMENTUM): results from an international, double-blind, randomised, controlled, phase 3 study. Lancet. 2023;401:269–80. https://doi.org/10.1016/S0140-6736(22)02036-0.
Gagelmann N, Hobbs GS, Campodonico E, Helbig G, Novak P, Schroeder T, et al. Splenic irradiation for myelofibrosis prior to hematopoietic cell transplantation: a global collaborative analysis. Am J Hematol. 2024;99:844–53.
Oechsler S, Gagelmann N, Wolschke C, Janson D, Badbaran A, Klyuchnikov E, et al. Graft-versus-host disease and impact on relapse in myelofibrosis undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 2024;59:550–7.
Kitanaka A, Takenaka K, Shide K, Miyamoto T, Kondo T, Ozawa K, et al. Splenic irradiation provides transient palliation for symptomatic splenomegaly associated with primary myelofibrosis: a report on 14 patients. Int J Hematol. 2016;103:423–8.
Kalman NS, Mukhopadhyay ND, Roberts CH, Chung HM, Clark WB, McCarty JM, et al. Low-dose splenic irradiation prior to hematopoietic cell transplantation in hypersplenic patients with myelofibrosis. Leuk Lymphoma. 2017;58:2983–4.
Bales JR, Kim HT, Portillo R, Patel C, McAfee S, Dey B, et al. Splenic irradiation prior to allogeneic hematopoietic cell transplantation for patients with myelofibrosis. Bone Marrow Transplant. 2023;58:459–61.
Campodonico E, Xue E, Piemontese S, Chiara A, Bruno A, Scorpio G, et al. Splenic irradiation prior to allogeneic transplant conditioning in myelofibrosis: a pilot experience. Curr Res Transl Med. 2023;71:103400.
Finazzi MC, Tefferi A, Rambaldi A. JAK inhibitor treatment‐resistant splenomegaly before transplantation in myelofibrosis: splenectomy or radiotherapy? Am J Hematol. 2024;99:804–5.
Ferreira Gomes G, Harrison C. Pelabresib (CPI-0610): an exciting novel drug for the treatment of myelofibrosis. Curr Hematol Malig Rep. 2023;18:113–20.
Pemmaraju N, Garcia JS, Perkins A, Harb JG, Souers AJ, Werner ME, et al. New era for myelofibrosis treatment with novel agents beyond Janus kinase‐inhibitor monotherapy: Focus on clinical development of BCL‐XL/BCL‐2 inhibition with navitoclax. Cancer 2023;129:3535–45.
Mascarenhas J, Kremyanskaya M, Patriarca A, Palandri F, Devos T, Passamonti F, et al. MANIFEST: pelabresib in combination with ruxolitinib for Janus kinase inhibitor treatment-naïve myelofibrosis. J Clin Oncol. 2023;41:4993–5004. https://doi.org/10.1200/JCO.22.01972.
Hernández‐Boluda J, Pastor‐Galán I, Arellano‐Rodrigo E, Raya J, Pérez‐Encinas M, Ayala R, et al. Predictors of thrombosis and bleeding in 1613 myelofibrosis patients from the Spanish Registry of Myelofibrosis. Br J Haematol. 2022;199:529–38.
Lavu S, Szuber N, Mudireddy M, Yogarajah M, Gangat N, Pardanani A, et al. Splanchnic vein thrombosis in patients with myeloproliferative neoplasms: the Mayo clinic experience with 84 consecutive cases. Am J Hematol. 2018;93:E61–4.
Sant’Antonio E, Guglielmelli P, Pieri L, Primignani M, Randi ML, Santarossa C, et al. Splanchnic vein thromboses associated with myeloproliferative neoplasms: An international, retrospective study on 518 cases. Am J Hematol. 2020;95:156–66.
Alvarez-Larrán A, Pereira A, Magaz M, Hernández-Boluda JC, Garrote M, Cuevas B, et al. Natural history of polycythemia vera and essential thrombocythemia presenting with splanchnic vein thrombosis. Ann Hematol. 2020;99:791–8.
Wong KM, Atenafu EG, Kim D, Kuruvilla J, Lipton JH, Messner H, et al. Incidence and risk factors for early hepatotoxicity and its impact on survival in patients with myelofibrosis undergoing allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:1589–99.
Silverstein MN. Gastrointestinal and abdominal manifestations of agnogenic myeloid metaplasia. Arch Intern Med. 1973;131:532.
Ward HP, Block MH. The natural history of agnogenic myeloid metaplasia (AMM) and a critical evaluation of its relationship with the myeloproliferative syndrome. Medicine. 1971;50:357–420.
Smallbone P, Sekhar M, Srour SA, Shpall EJ, Popat UR. Hematopoietic stem cell transplantation in patients with myelofibrosis and splanchnic vein thrombosis. Transplant Cell Ther. 2024;30:S103.
Polverelli N, Farina M, D’Adda M, Damiani E, Grazioli L, Leoni A, et al. How we manage myelofibrosis candidates for allogeneic stem cell transplantation. Cells 2022;11:553.
Tan HK, Leow WQ, Chang PE. Ruxolitinib for the treatment of portal hypertension in a patient with primary myelofibrosis. Gastroenterology 2019;157:e26–7.
Koschmieder S, Koppelle A, Seifert H. Ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:2031–5.
Yan M, Geyer H, Mesa R, Atallah E, Callum J, Bartoszko J, et al. Clinical features of patients with philadelphia-negative myeloproliferative neoplasms complicated by portal hypertension. Clin Lymphoma Myeloma Leuk. 2015;15:e1–5.
Lee J, Sung PS, Eom KS, Yang H, Lee SK, Bwa AH, et al. Clinical characteristics of portal hypertension complicated by gastroesophageal varices in patients with myeloproliferative neoplasms. Clin Mol Hepatol. 2020;26:78–82.
Pieri L, Paoli C, Arena U, Marra F, Mori F, Zucchini M, et al. Safety and efficacy of ruxolitinib in splanchnic vein thrombosis associated with myeloproliferative neoplasms. Am J Hematol. 2017;92:187–95.
Verstovsek S, Atallah E, Mascarenhas J, Sun H, Montgomery M, Gupta V, et al. Efficacy of ruxolitinib on hepatomegaly in patients with myelofibrosis. Leukemia 2016;30:1413–5.
Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022;43:3618–731.
Garcia-Manero G, Schuster SJ, Patrick H, Martinez J. Pulmonary hypertension in patients with myelofibrosis secondary to myeloproliferative diseases. Am J Hematol. 1999;60:130–5.
Cortelezzi A, Gritti G, Del Papa N, Pasquini MC, Calori R, Gianelli U, et al. Pulmonary arterial hypertension in primary myelofibrosis is common and associated with an altered angiogenic status. Leukemia 2008;22:646–9.
Lopez-Mattei J, Verstovsek S, Fellman B, Iliescu C, Bhatti K, Hassan SA, et al. Prevalence of pulmonary hypertension in myelofibrosis. Ann Hematol. 2020;99:781–9.
Brabrand M, Hansen KN, Laursen CB, Larsen TS, Vestergaard H, Abildgaard N. Frequency and etiology of pulmonary hypertension in patients with myeloproliferative neoplasms. Eur J Haematol. 2019;102:227–34.
Gupta R, Jamal F, Yang D, Chendri C, Aldoss I, Malki M, Al, et al. Pulmonary hypertension is associated with increased nonrelapse mortality after allogeneic hematopoietic cell transplantation for myelofibrosis. Bone Marrow Transplant. 2020;55:877–83.
Salit RB, Baker KK, Edwards R, Tobin G, Kicska G, Gooley TA, et al. Diagnosis of pulmonary hypertension by noninvasive methods in hematopoietic cell transplant patients with myelofibrosis. Bone Marrow Transplant. 2020;55:1681–3.
Perram J, Ross DM, McLornan D, Gowin K, Kröger N, Gupta V, et al. Innovative strategies to improve hematopoietic stem cell transplant outcomes in myelofibrosis. Am J Hematol. 2022;97:1464–77.
Steensma DP, Hook CC, Stafford SL, Tefferi A. Low‐dose, single‐fraction, whole‐lung radiotherapy for pulmonary hypertension associated with myelofibrosis with myeloid metaplasia. Br J Haematol. 2002;118:813–6.
Chunduri S, Gaitonde S, Ciurea SO, Hoffman R, Rondelli D. Pulmonary extramedullary hematopoiesis in patients with myelofibrosis undergoing allogeneic stem cell transplantation. Haematologica 2008;93:1593–5.
Maccaferri M, Leonardi G, Marasca R, Colaci E, Paolini A, Soci F, et al. Ruxolitinib for pulmonary extramedullary hematopoiesis in myelofibrosis. Leuk Lymphoma. 2014;55:2207–8.
Fujimoto A, Hamaguchi S, Suzuki R. Case of pulmonary extramedullary hematopoiesis responding to ruxolitinib. Leuk Res Rep. 2022;17:100290.
Tabarroki A, Lindner DJ, Visconte V, Zhang L, Rogers HJ, Parker Y, et al. Ruxolitinib leads to improvement of pulmonary hypertension in patients with myelofibrosis. Leukemia 2014;28:1486–93.
Gerds AT, Bose P, Hobbs GS, Kuykendall AT, Neilson LM, Song J, et al. Treating anemic patients with myelofibrosis in the new Janus kinase inhibitor era: current evidence and real-world implications. HemaSphere. 2022;6. https://journals.lww.com/hemasphere/fulltext/2022/10000/treating_anemic_patients_with_myelofibrosis_in_the.10.aspx.
Palandri F, Breccia M, Mazzoni C, Auteri G, Elli EM, Trawinska MM, et al. Ruxolitinib in cytopenic myelofibrosis: response, toxicity, drug discontinuation, and outcome. Cancer. 2023;129:1704–13. https://doi.org/10.1002/cncr.34722.
Huang J, Li CY, Mesa RA, Wu W, Hanson CA, Pardanani A, et al. Risk factors for leukemic transformation in patients with primary myelofibrosis. Cancer. 2008;112:2726–32. https://doi.org/10.1002/cncr.23505.
Winton EF, Kota V. Momelotinib in myelofibrosis: JAK1/2 inhibitor with a role in treating and understanding the anemia. Future Oncol. 2016;13:395–407. https://doi.org/10.2217/fon-2016-0417.
Bacigalupo A, Soraru M, Dominietto A, Pozzi S, Geroldi S, Van Lint MT, et al. Allogeneic hemopoietic SCT for patients with primary myelofibrosis: a predictive transplant score based on transfusion requirement, spleen size and donor type. Bone Marrow Transplant. 2010;45:458–63.
Gerds AT, Vannucchi AM, Passamonti F, Kremyanskaya M, Gotlib JR, Palmer JM, et al. SIMPLIFY-1: a phase 2 study of luspatercept in patients with myelofibrosis-associated anemia. Blood. 2019;134:557 https://doi.org/10.1182/blood-2019-122546.
Harrison CN, Vannucchi AM, Platzbecker U, Cervantes F, Gupta V, Lavie D. et al. Momelotinib versus best available therapy in patients with myelofibrosis previously treated with ruxolitinib (SIMPLIFY 2): a randomised, open-label, phase 3 trial. Lancet Haematol. 2018;5:e73–81. https://doi.org/10.1016/S2352-3026(17)30237-5.
Mascarenhas J, Komrokji RS, Palandri F, Martino B, Niederwieser D, Reiter A, et al. Randomized, single-blind, multicenter phase ii study of two doses of imetelstat in relapsed or refractory myelofibrosis. J Clin Oncol. 2021;39:2881–92. https://doi.org/10.1200/JCO.20.02864.
Gerds AT, Savona MR, Scott BL, Talpaz M, Egyed M, Harrison CN, et al. Determining the recommended dose of pacritinib: results from the PAC203 dose-finding trial in advanced myelofibrosis. Blood Adv. 2020;4:5825–35. https://doi.org/10.1182/bloodadvances.2020003314.
Beauverd Y, McLornan DP, Harrison CN. Pacritinib: a new agent for the management of myelofibrosis. Expert Opin Pharmacother. 2015;16:2381–90. https://doi.org/10.1517/14656566.2015.1088831.
Rampal RK, Grosicki S, Chraniuk D, Abruzzese E, Bose P, Gerds AT. et al. Pelabresib in combination with ruxolitinib for Janus kinase inhibitor treatment-naïve patients with myelofibrosis: results of the MANIFEST-2 randomized, double-blind, phase 3 study. Blood. 2023;142:628. https://doi.org/10.1182/blood-2023-179141.
Mascarenhas J, Harrison C, Luptakova K, Christo J, Wang J, Mertz JA. et al. MANIFEST-2, a global, phase 3, randomized, double-blind, active-control study of cpi-0610 and ruxolitinib vs. placebo and ruxolitinib in JAK-inhibitor-naive myelofibrosis patients. Blood. 2020;136:43. https://doi.org/10.1182/blood-2020-140901.
Barba P, Valcárcel D, Pérez-Simón JA, Fernández-Avilés F, Piñana JL, Martino R. et al. Impact of hyperferritinemia on the outcome of reduced-intensity conditioning allogeneic hematopoietic cell transplantation for lymphoid malignancies. Biol Blood Marrow Transplant. 2013;19:597–601. https://www.sciencedirect.com/science/article/pii/S1083879112011779.
Armand P, Kim HT, Cutler CS, Ho VT, Koreth J, Alyea EP, et al. Prognostic impact of elevated pretransplantation serum ferritin in patients undergoing myeloablative stem cell transplantation. Blood. 2007;109:4586–8. https://doi.org/10.1182/blood-2006-10-054924.
Yan Z, Chen X, Wang H, Chen Y, Chen L, Wu P, et al. Effect of pre-transplantation serum ferritin on outcomes in patients undergoing allogeneic hematopoietic stem cell transplantation: a meta-analysis. Medicine. 2018;97. https://journals.lww.com/md-journal/fulltext/2018/07060/effect_of_pre_transplantation_serum_ferritin_on.1.aspx.
Atilla E, Toprak SK, Demirer T. Current review of iron overload and related complications in hematopoietic stem cell transplantation. Turkish J Hematol. 2017;34:1–9.
Elli EM, Di Veroli A, Bartoletti D, Iurlo A, Carmosino I, Benevolo G, et al. Deferasirox in the management of iron overload in patients with myelofibrosis treated with ruxolitinib: the multicentre retrospective RUX‐IOL study. Br J Haematol. 2022;197:190–200.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9.
Raimondi R, Tosetto A, Oneto R, Cavazzina R, Rodeghiero F, Bacigalupo A, et al. Validation of the hematopoietic cell transplantation-specific comorbidity index: a prospective, multicenter GITMO study. Blood. 2012;120:1327–33.
Gagelmann N, Ditschkowski M, Bogdanov R, Bredin S, Robin M, Cassinat B, et al. Comprehensive clinical-molecular transplant scoring system for myelofibrosis undergoing stem cell transplantation. Blood. 2019;133:2233–42.
Tamari R, McLornan DP, Ahn KW, Estrada-Merly N, Hernández-Boluda JC, Giralt S, et al. A simple prognostic system in patients with myelofibrosis undergoing allogeneic stem cell transplantation: a CIBMTR/EBMT analysis. Blood Adv. 2023;7:3993–4002.
Hernández-Boluda JC, Pereira A, Alvarez-Larran A, Martín AA, Benzaquen A, Aguirre L, et al. Predicting survival after allogeneic hematopoietic cell transplantation in myelofibrosis: performance of the Myelofibrosis Transplant Scoring System (MTSS) and development of a new prognostic model. Biol Blood Marrow Transplant. 2020;26:2237–44.
Doney K, McMillen K, Buono L, Deeg HJ, Gooley T. Impact of body mass index on outcomes of hematopoietic stem cell transplantation in adults. Biol Blood Marrow Transplant. 2019;25:613–20.
Hernández‐Boluda J, Pereira A, Kröger N, Cornelissen JJ, Finke J, Beelen D, et al. Allogeneic hematopoietic cell transplantation in older myelofibrosis patients: a study of the chronic malignancies working party of EBMT and the Spanish Myelofibrosis Registry. Am J Hematol. 2021;96:1186–94.
Jayani RV. How old is too old? Frailty and geriatric assessments of older patients undergoing allogeneic HCT. Hematology 2023;2023:709–14.
Polverelli N, Tura P, Battipaglia G, Malagola M, Bernardi S, Gandolfi L, et al. Multidimensional geriatric assessment for elderly hematological patients (≥60 years) submitted to allogeneic stem cell transplantation. a French-Italian 10-year experience on 228 patients. Bone Marrow Transplant. 2020;55:2224–33.
Muffly LS, Boulukos M, Swanson K, Kocherginsky M, Cerro P, del, Schroeder L, et al. Pilot study of comprehensive geriatric assessment (CGA) in allogeneic transplant: CGA captures a high prevalence of vulnerabilities in older transplant recipients. Biol Blood Marrow Transplant. 2013;19:429–34.
Muffly LS, Kocherginsky M, Stock W, Chu Q, Bishop MR, Godley LA, et al. Geriatric assessment to predict survival in older allogeneic hematopoietic cell transplantation recipients. Haematologica 2014;99:1373–9.
Kneis S, Straub E, Walz ID, von Olshausen P, Wehrle A, Gollhofer A, et al. Gait analysis of patients after allogeneic hematopoietic cell transplantation reveals impairments of functional performance. Integr Cancer Ther. 2020;19:153473542091578.
Smith PJ, Lew M, Lowder Y, Romero K, Thompson JC, Bohannon L, et al. Cognitive impairment in candidates for allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2022;57:89–94.
Sorror ML, Gooley TA, Storer BE, Gerds AT, Sekeres MA, Medeiros BC. et al. An 8-year pragmatic observation evaluation of the benefits of allogeneic HCT in older and medically infirm patients with AML. Blood. 2023;141:295–308.
Salas MQ, Atenafu EG, Pasic I, Bascom O, Wilson L, Lam W, et al. HCT frailty scale for younger and older adults undergoing allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2023;58:1237–46.
Funke VAM, Bonfim C, Darrigo Juniro LG, Barroso Duarte F. Access to hematopoietic stem cell transplantation in Brazil: facing our challenges. J Bone Marrow Transplant Cell Ther. 2023;4:211.
Moreira Funke VA, Lima ACM, Hamerschlak N, Rensi Colturato VA, De Souza MP, Vigorito AC, et al. DIPSS score validation as a tool to estimate survival and non-relapse mortality after hematopoietic stem cell transplant in a Brazilian multicentric cohort. Transplant Cell Ther. 2022;28:S131–3.
Simione AJ, da Silva CC, da Sabaini PM, Macedo AV, das Neves HRA, da Geraldo BL. et al. Current use and outcomes of hematopoietic stem cell transplantation: Brazilian summary slides—2024. J Bone Marrow Transplant Cell Ther. 2024;5:228.
Singh S, Cao Q, Demorest C, He F, Kramer A, Holtan S, et al. The prevalence of pretransplant frailty and mental distress in hematopoietic cell transplantation and association with clinical outcomes. Transplant Cell Ther. 2024;30:919.e1–9.
Sung AD, Koll T, Gier SH, Racioppi A, White G, Lew M. et al. Preconditioning frailty phenotype influences survival and relapse for older allogeneic transplantation recipients. Transplant Cell Ther. 2024;30:415.e1–16.
Huang LW, Shi Y, Andreadis C, Logan AC, Mannis GN, Smith CC, et al. Association of geriatric measures and global frailty with cognitive decline after allogeneic hematopoietic cell transplantation in older adults. J Geriatr Oncol 2023;14:101623.
Ombres R, des Bordes JKA, Popat UR, Yennu S, Champlin RE, Mohile SG. et al. Serial frailty assessments following allogeneic stem cell transplant in older adults: a pilot study. J Geriatr Oncol. 2022;13:194–9.
Rodrigues M, de Souza PMR, de Oliveira Muniz Koch L, Hamerschlak N. The use of comprehensive geriatric assessment in older patients before allologeneic hematopoietic stem cell transplantation: a cross-sectional study. J Geriatr Oncol. 2020;11:100–6.
Lin RJ, Elko TA, Devlin SM, Shahrokni A, Jakubowski AA, Dahi PB, et al. Impact of geriatric vulnerabilities on allogeneic hematopoietic cell transplantation outcomes in older patients with hematologic malignancies. Bone Marrow Transplant. 2020;55:157–64.
Acknowledgements
We would like to thank Juan Carlos García Pagán (Hepatology Unit, Hospital Clínic, Barcelona) and Enrique Santas (Cardiology Department, Hospital Clínico, Valencia) for kindly reviewing the present manuscript.
Author information
Authors and Affiliations
Contributions
NP, JCHB, NC, CG, MM, FBD, VAM, CZ, DPM wrote and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Polverelli, N., Hernández-Boluda, J.C., Gagelmann, N. et al. Navigating ‘grey areas’ and challenges during evaluation of transplant eligibility in specific myelofibrosis populations: a perspective on behalf of the Chronic Malignancies Working Party of the EBMT. Bone Marrow Transplant 60, 10–18 (2025). https://doi.org/10.1038/s41409-024-02437-6
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41409-024-02437-6