Abstract
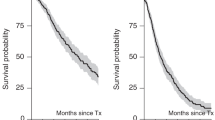
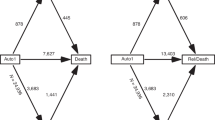
Autologous hematopoietic cell transplants (auto-HCTs) remain the standard of care for transplant-eligible MM patients. The general practice has been to undergo upfront apheresis following induction to collect sufficient number of CD34+ cells to facilitate two auto-HCTs. However, 5–30% of MM patients do not initially mobilise a sufficient number of hematopoietic stem cells and are classified as poor mobilizers (PM). We compared the baseline characteristics and outcomes of 61 PMs and 816 non-PM patients who underwent a second auto-HCT and who were enrolled in the non-interventional CALM study (NCT01362972). Only patients who collected CD34+ prior to auto-HCT1 were included. Auto-HCT2 comprised both tandem and salvage transplants. PMs were re-mobilized with plerixafor (n = 24, 39.3%) or non-plerixafor-based regimens (n = 37, 60.7%). There were no significant differences in engraftment, progression-free survival (PFS) or overall survival (OS) after the second auto-HCT between PM and non-PM patients. There was a trend to shorter PFS in PM patients undergoing salvage auto-HCT (median 9.6 vs. 12.9 months; p = 0.08) but no significant difference in OS. The median OS was 41.1 months for PM and 41.2 months for non-PM patients (p = 0.86). These data suggest that salvage mobilization is effective and does not affect overall outcomes after a second auto-HCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Dimopoulos MA, Moreau P, Terpos E, Mateos MV, Zweegman S, Cook G, et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32:309–22.
Cavo M, Gay F, Beksac M, Pantani L, Petrucci MT, Dimopoulos MA, et al. Autologous haematopoietic stem-cell transplantation versus bortezomib–melphalan–prednisone, with or without bortezomib–lenalidomide–dexamethasone consolidation therapy, and lenalidomide maintenance for newly diagnosed multiple myeloma (EMN02/HO95): a multicentre, randomised, open-label, phase 3 study. Lancet Haematol. 2020;7:e456–e468.
Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N Engl J Med. 2017;376:1311–20.
Richardson PG, Jacobus SJ, Weller EA, Hassoun H, Lonial S, Raje NS, et al. Triplet Therapy, Transplantation, and Maintenance until Progression in Myeloma. N Engl J Med. 2022;387:132–47.
Cook G, Williams C, Brown JM, Cairns DA, Cavenagh J, Snowden JA, et al. High-dose chemotherapy plus autologous stem-cell transplantation as consolidation therapy in patients with relapsed multiple myeloma after previous autologous stem-cell transplantation (NCRI Myeloma X Relapse [Intensive trial]): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:874–85.
Cook G, Ashcroft AJ, Cairns DA, Williams CD, Brown JM, Cavenagh JD, et al. The effect of salvage autologous stem-cell transplantation on overall survival in patients with relapsed multiple myeloma (final results from BSBMT/UKMF Myeloma X Relapse [Intensive]): a randomised, open-label, phase 3 trial. Lancet Haematol. 2016;3:e340–e351.
Cook G, Royle K, O’Connor S, Cairns DA, Ashcroft AJ, Williams CD, et al. The impact of cytogenetics on duration of response and overall survival in patients with relapsed multiple myeloma (long‐term follow‐up results from BSBMT / UKMF Myeloma X Relapse [Intensive]): a randomised, open‐label, phasetrial. Br J Haematol. 2019;185:450–67.
Goldschmidt H, Baertsch M-A, Schlenzka J, Becker N, Habermehl C, Hielscher T, et al. Salvage autologous transplant and lenalidomide maintenance vs. lenalidomide/dexamethasone for relapsed multiple myeloma: the randomized GMMG phase III trial ReLApsE. Leukemia. 2021;35:1134–44.
Gagelmann N, Eikema D-J, Koster L, Caillot D, Pioltelli P, Lleonart JB, et al. Tandem Autologous Stem Cell Transplantation Improves Outcomes in Newly Diagnosed Multiple Myeloma with Extramedullary Disease and High-Risk Cytogenetics: A Study from the Chronic Malignancies Working Party of the European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transpl. 2019;25:2134–42.
Drozd-Sokołowska J, Gras L, Zinger N, Snowden JA, Arat M, Basak G, et al. Autologous hematopoietic cell transplantation for relapsed multiple myeloma performed with cells procured after previous transplantation–study on behalf of CMWP of the EBMT. Bone Marrow Transpl. 2022;57:633–40.
Davis JA, Sborov DW, Wesson W, Julian K, Abdallah A-O, McGuirk JP, et al. Efficacy and Safety of CD34+ Stem Cell Boost for Delayed Hematopoietic Recovery After BCMA Directed CAR T-cell Therapy. Transpl Cell Ther. 2023;29:567–71.
Mohan M, Szabo A, Patwari A, Esselmann J, Patel T, Bachu R, et al. Autologous stem cell boost improves persistent immune effector cell associated hematotoxicity following BCMA directed chimeric antigen receptor T (CAR T) cell therapy in multiple myeloma. Bone Marrow Transpl. 2024;59:647–52.
Mohty M, Hübel K, Kröger N, Aljurf M, Apperley J, Basak GW, et al. Autologous haematopoietic stem cell mobilisation in multiple myeloma and lymphoma patients: a position statement from the European Group for Blood and Marrow Transplantation. Bone Marrow Transpl. 2014;49:865–72.
Czerw T, Sadus-Wojciechowska M, Michalak K, Najda J, Mendrek W, Sobczyk-Kruszelnicka M, et al. Increased Efficacy of Stem Cell Chemomobilization with Intermediate-Dose Cytarabine Plus Granulocyte Colony-Stimulating Factor (G-CSF) Compared with G-CSF Alone in Patients with Multiple Myeloma: Results of a Randomized Trial. Biol Blood Marrow Transpl. 2019;25:248–55.
Dhakal B, Zhang M-J, Burns LJ, Tang X, Meyer C, Mau L-W, et al. Efficacy, safety, and cost of mobilization strategies in multiple myeloma: a prospective, observational study. Haematologica. 2023;108:2249–54.
Giralt S, Costa L, Schriber J, Dipersio J, Maziarz R, McCarty J, et al. Optimizing autologous stem cell mobilization strategies to improve patient outcomes: consensus guidelines and recommendations. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2014;20:295–308.
Wuchter P, Ran D, Bruckner T, Schmitt T, Witzens-Harig M, Neben K, et al. Poor Mobilization of Hematopoietic Stem Cells—Definitions, Incidence, Risk Factors, and Impact on Outcome of Autologous Transplantation. Biol Blood Marrow Transpl. 2010;16:490–9.
Ozsan GH, Micallef IN, Dispenzieri A, Kumar S, Lacy MQ, Dingli D, et al. Hematopoietic recovery kinetics predicts for poor CD34+ cell mobilization after cyclophosphamide chemotherapy in multiple myeloma. Am J Hematol. 2012;87:1–4.
Perseghin P, Terruzzi E, Dassi M, Baldini V, Parma M, Coluccia P, et al. Management of poor peripheral blood stem cell mobilization: Incidence, predictive factors, alternative strategies and outcome. A retrospective analysis on 2177 patients from three major Italian institutions. Transfus Apher Sci. 2009;41:33–37.
DiPersio JF, Stadtmauer EA, Nademanee A, Micallef INM, Stiff PJ, Kaufman JL, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113:5720–6.
Milone G, Martino M, Spadaro A, Leotta S, Di Marco A, Scalzulli P, et al. Plerixafor on-demand combined with chemotherapy and granulocyte colony-stimulating factor: significant improvement in peripheral blood stem cells mobilization and harvest with no increase in costs. Br J Haematol. 2014;164:113–23.
Farina L, Guidetti A, Spina F, Roncari L, Longoni P, Ravagnani F, et al. Plerixafor ‘on demand’: results of a strategy based on peripheral blood CD34+ cells in lymphoma patients at first or subsequent mobilization with chemotherapy+G-CSF. Bone Marrow Transpl. 2014;49:453–5.
Hübel K, Fresen MM, Apperley JF, Basak GW, Douglas KW, Gabriel IH, et al. European data on stem cell mobilization with plerixafor in non-Hodgkin’s lymphoma, Hodgkin’s lymphoma and multiple myeloma patients. A subgroup analysis of the European Consortium of stem cell mobilization. Bone Marrow Transpl. 2012;47:1046–50.
Olivieri A, Marchetti M, Lemoli R, Tarella C, Iacone A, Lanza F, et al. Proposed definition of ‘poor mobilizer’ in lymphoma and multiple myeloma: an analytic hierarchy process by ad hoc working group Gruppo ItalianoTrapianto di Midollo Osseo. Bone Marrow Transpl. 2012;47:342–51.
Basak GW, Mikala G, Koristek Z, Jaksic O, Basic-Kinda S, Cegledi A, et al. Plerixafor to rescue failing chemotherapy-based stem cell mobilization: it’s not too late. Leuk Lymphoma. 2011;52:1711–9.
Nahi H, Celanovic M, Liu Q, Lund J, Peceliunas V. A Pilot, Exploratory, Randomized, Phase II Safety Study Evaluating Tumor Cell Mobilization and Apheresis Product Contamination in Patients Treated with Granulocyte Colony-Stimulating Factor Alone or Plus Plerixafor. Biol Blood Marrow Transpl. 2019;25:34–40.
Micallef IN, Stiff PJ, Nademanee AP, Maziarz RT, Horwitz ME, Stadtmauer EA, et al. Plerixafor Plus Granulocyte Colony-Stimulating Factor for Patients with Non-Hodgkin Lymphoma and Multiple Myeloma: Long-Term Follow-Up Report. Biol Blood Marrow Transpl. 2018;24:1187–95.
Morris C, Chabannon C, Masszi T, Russell N, Nahi H, Kobbe G, et al. Results from a multicenter, noninterventional registry study for multiple myeloma patients who received stem cell mobilization regimens with and without plerixafor. Bone Marrow Transpl. 2020;55:356–66.
Durie BGM, Harousseau J-L, Miguel JS, Bladé J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73.
R Core Team. A language and environment for statistical computing. R Foundation for Statistical Computing. 2022. Available from: https://www.r-project.org/.
Olivieri A, Saraceni F, De Luca A. Focus on: Prognostic scores to predict stem cell mobilization. Transfus Apher Sci. 2024;63:103935.
Sauer S, Hieke L, Brandt J, Müller-Tidow C, Schmitt A, Kauer J, et al. Impact of Clinical Parameters and Induction Regimens on Peripheral Blood Stem-Cell Mobilization and Collection in Multiple Myeloma Patients. Transfus Med Hemotherapy. 2023;50:382–95.
Moreb JS, Byrne M, Shugarman I, Zou F, Xiong S, May WS, et al. Poor peripheral blood stem cell mobilization affects long‐term outcomes in multiple myeloma patients undergoing autologous stem cell transplantation. J Clin Apheresis. 2018;33:29–37.
Hsu T-L, Tsai C-K, Liu C-Y, Yeh C-M, Lin F-L, Hsiao L-T, et al. Risk Factors and Outcomes of Stem Cell Mobilization Failure in Multiple Myeloma Patients. Transfus Med Hemotherapy. 2023;50:39–50.
Hagen PA, Stiff P. The Role of Salvage Second Autologous Hematopoietic Cell Transplantation in Relapsed Multiple Myeloma. Biol Blood Marrow Transpl. 2019;25:e98–e107.
Kumar S, Giralt S, Stadtmauer EA, Harousseau JL, Palumbo A, Bensinger W, et al. Mobilization in myeloma revisited: IMWG consensus perspectives on stem cell collection following initial therapy with thalidomide-, lenalidomide-, or bortezomib-containing regimens. Blood. 2009;114:1729–35.
Tricot G, Cottler-Fox MH, Calandra G. Safety and efficacy assessment of plerixafor in patients with multiple myeloma proven or predicted to be poor mobilizers, including assessment of tumor cell mobilization. Bone Marrow Transpl. 2010;45:63–68.
DiPersio JF, Ho AD, Hanrahan J, Hsu FJ, Fruehauf S. Relevance and Clinical Implications of Tumor Cell Mobilization in the Autologous Transplant Setting. Biol Blood Marrow Transpl. 2011;17:943–55.
Acknowledgements
We are indebted to all centres participating to the EBMT database, and especially the ones who contributed to this study.
Author information
Authors and Affiliations
Contributions
MS designed the study and wrote the initial manuscript. JDS, DML and LG wrote the final version of the manuscript. LK managed data, LG did the statistics. DML, CM and PH supervised the study. LK, FF, SM, RF, GB, JA, JB, AR, MR, ME, MT, DB, SL, CI, JP, AP, IS, SS, CM, MB, KR, PH critically revised the paper and approved the submitted and final version.
Corresponding author
Ethics declarations
Competing interests
JDS – honoraria AbbVie, Roche, Janssen, Astra Zeneca, SOBI, Takeda, BMS, Novartis, Swixx; Advisory board meetings organized by Janssen-Cilag, Sanofi, AstraZeneca, Roche, BeiGene; AP - honoraria from Behring and Abbvie, participation in scientific Advisory board meetings organized by Abbvie, Janssen-Cilag, Novartis, Pfizer, Sanofi and Takeda; none of COI directly related to the study.
Ethics approval
This retrospective study was approved by the Chronic Malignancies Working Party (CMWP) of the EBMT and was performed in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sever, M., Drozd-Sokolowska, J., Gras, L. et al. Satisfactory outcomes following a second autologous hematopoietic cell transplantation for multiple myeloma in poor stem cell mobilizers: a retrospective study on behalf of the Chronic Malignancies Working Party of the EBMT. Bone Marrow Transplant 60, 211–219 (2025). https://doi.org/10.1038/s41409-024-02460-7
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41409-024-02460-7