Abstract
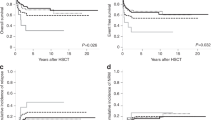
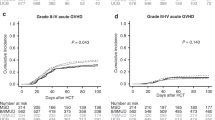
Patients with TP53-mutated myelodysplastic neoplasms (MDS) have unfavorable prognoses; the benefit of cytoreductive treatment before hematopoietic stem cell transplantation (HSCT) is debated. We retrospectively analyzed 284 MDS patients undergoing allogeneic HSCT; among which 49 had TP53 mutation, with 38 receiving cytoreduction and 11 treated exclusively with best supportive care (BSC) before transplantation. Regardless of TP53 allelic state, patients with mutated-TP53 had a lower overall survival rate and higher relapse rate than those with wild-type TP53 (P < 0.001, P = 0.002, respectively). Among the TP53-mutated cohort, the 2-year overall survival rate in the cytoreduction group was comparable to that in the BSC group (34.6% vs. 45.5%, P = 0.53), and no other prognostic benefit was observed as well (all P < 0.05). Moreover, no prognostic difference was found among the chemotherapy subgroup, hypomethylating agent subgroup, and BSC subgroup (all P > 0.05). Patients in the pre-HSCT measurable residual disease (MRD) negative subgroup, pre-HSCT MRD-positive subgroup, and BSC subgroup exhibited similar prognoses (all P > 0.05). Multivariate analyses showed that pre-HSCT cytoreduction was not associated with post-transplant survival (all P > 0.05). In conclusion, TP53-mutated MDS patients have poor post-HSCT outcomes; compared to BSC, pre-HSCT cytoreduction doesn’t improve prognosis, even in those with MRD negative before transplantation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data used and analyzed are available from the corresponding author upon reasonable request.
References
Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36:1703–19. https://doi.org/10.1038/s41375-022-01613-1.
Cazzola M. Myelodysplastic syndromes. N Engl J Med. 2020;383:1358–74. https://doi.org/10.1056/NEJMra1904794.
Bejar R, Stevenson K, Abdel-Wahab O, Galili N, Nilsson B, Garcia-Manero G, et al. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med. 2011;364:2496–506. https://doi.org/10.1056/NEJMoa1013343.
Haferlach T, Nagata Y, Grossmann V, Okuno Y, Bacher U, Nagae G, et al. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia. 2014;28:241–7. https://doi.org/10.1038/leu.2013.336.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. https://doi.org/10.1016/s0092-8674(00)81683-9.
Levine AJ. p53: 800 million years of evolution and 40 years of discovery. Nat Rev Cancer. 2020;20:471–80. https://doi.org/10.1038/s41568-020-0262-1.
Bejar R, Stevenson KE, Caughey B, Lindsley RC, Mar BG, Stojanov P, et al. Somatic mutations predict poor outcome in patients with myelodysplastic syndrome after hematopoietic stem-cell transplantation. J Clin Oncol. 2014;32:2691–8. https://doi.org/10.1200/JCO.2013.52.3381.
Della Porta MG, Galli A, Bacigalupo A, Zibellini S, Bernardi M, Rizzo E, et al. Clinical effects of driver somatic mutations on the outcomes of patients with myelodysplastic syndromes treated with allogeneic hematopoietic stem-cell transplantation. J Clin Oncol. 2016;34:3627–37. https://doi.org/10.1200/JCO.2016.67.3616.
Versluis J, Saber W, Tsai HK, Gibson CJ, Dillon LW, Mishra A, et al. Allogeneic hematopoietic cell transplantation improves outcome in myelodysplastic syndrome across high-risk genetic subgroups: genetic analysis of the blood and marrow transplant clinical trials network 1102 study. J Clin Oncol. 2023;41:4497–510. https://doi.org/10.1200/JCO.23.00866.
de Witte T, Bowen D, Robin M, Malcovati L, Niederwieser D, Yakoub-Agha I, et al. Allogeneic hematopoietic stem cell transplantation for MDS and CMML: recommendations from an international expert panel. Blood. 2017;129:1753–62. https://doi.org/10.1182/blood-2016-06-724500.
Sun YQ, Xu LP, Liu KY, Zhang XH, Yan CH, Jin J, et al. Pre-transplantation cytoreduction does not benefit advanced myelodysplastic syndrome patients after myeloablative transplantation with grafts from family donors. Cancer Commun. 2021;41:333–44. https://doi.org/10.1002/cac2.12140.
Schroeder T, Wegener N, Lauseker M, Rautenberg C, Nachtkamp K, Schuler E, et al. Comparison between upfront transplantation and different pretransplant cytoreductive treatment approaches in patients with high-risk myelodysplastic syndrome and secondary acute myelogenous leukemia. Biol Blood Marrow Transpl. 2019;25:1550–9. https://doi.org/10.1016/j.bbmt.2019.03.011.
Bernard E, Tuechler H, Greenberg PL, Hasserjian RP, Arango Ossa JE, Nannya Y, et al. Molecular international prognostic scoring system for myelodysplastic syndromes. NEJM Evid. 2022. https://doi.org/10.1056/EVIDoa2200008.
Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Sole F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–65. https://doi.org/10.1182/blood-2012-03-420489.
Malcovati L, Della Porta MG, Strupp C, Ambaglio I, Kuendgen A, Nachtkamp K, et al. Impact of the degree of anemia on the outcome of patients with myelodysplastic syndrome and its integration into the WHO classification-based Prognostic Scoring System (WPSS). Haematologica. 2011;96:1433–40. https://doi.org/10.3324/haematol.2011.044602.
Weinberg OK, Siddon A, Madanat YF, Gagan J, Arber DA, Dal Cin P, et al. TP53 mutation defines a unique subgroup within complex karyotype de novo and therapy-related MDS/AML. Blood Adv. 2022;6:2847–53. https://doi.org/10.1182/bloodadvances.2021006239.
Luo Y, Xiao H, Lai X, Shi J, Tan Y, He J, et al. T-cell-replete haploidentical HSCT with low-dose anti-T-lymphocyte globulin compared with matched sibling HSCT and unrelated HSCT. Blood. 2014;124:2735–43. https://doi.org/10.1182/blood-2014-04-571570.
Malard F, Holler E, Sandmaier BM, Huang H, Mohty M. Acute graft-versus-host disease. Nat Rev Dis Prim. 2023;9:27. https://doi.org/10.1038/s41572-023-00438-1.
Lee SJ. Classification systems for chronic graft-versus-host disease. Blood. 2017;129:30–37. https://doi.org/10.1182/blood-2016-07-686642.
Holtan SG, DeFor TE, Lazaryan A, Bejanyan N, Arora M, Brunstein CG, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125:1333–8. https://doi.org/10.1182/blood-2014-10-609032.
Ruggeri A, Labopin M, Ciceri F, Mohty M, Nagler A. Definition of GvHD-free, relapse-free survival for registry-based studies: an ALWP-EBMT analysis on patients with AML in remission. Bone Marrow Transpl. 2016;51:610–1. https://doi.org/10.1038/bmt.2015.305.
Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka HM, et al. International consensus classification of myeloid neoplasms and acute leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140:1200–28. https://doi.org/10.1182/blood.2022015850.
Zhao Y, Chen W, Yu J, Pei S, Zhang Q, Shi J, et al. TP53 in MDS and AML: biological and clinical advances. Cancer Lett. 2024;588:216767 https://doi.org/10.1016/j.canlet.2024.216767.
Haase D, Germing U, Schanz J, Pfeilstocker M, Nosslinger T, Hildebrandt B, et al. New insights into the prognostic impact of the karyotype in MDS and correlation with subtypes: evidence from a core dataset of 2124 patients. Blood. 2007;110:4385–95. https://doi.org/10.1182/blood-2007-03-082404.
Hunter AM, Komrokji RS, Yun S, Al Ali N, Chan O, Song J, et al. Baseline and serial molecular profiling predicts outcomes with hypomethylating agents in myelodysplastic syndromes. Blood Adv. 2021;5:1017–28. https://doi.org/10.1182/bloodadvances.2020003508.
Yoshizato T, Nannya Y, Atsuta Y, Shiozawa Y, Iijima-Yamashita Y, Yoshida K, et al. Genetic abnormalities in myelodysplasia and secondary acute myeloid leukemia: impact on outcome of stem cell transplantation. Blood. 2017;129:2347–58. https://doi.org/10.1182/blood-2016-12-754796.
Lindsley RC, Saber W, Mar BG, Redd R, Wang T, Haagenson MD, et al. Prognostic mutations in myelodysplastic syndrome after stem-cell transplantation. N Engl J Med. 2017;376:536–47. https://doi.org/10.1056/NEJMoa1611604.
Bernard E, Nannya Y, Hasserjian RP, Devlin SM, Tuechler H, Medina-Martinez JS, et al. Implications of TP53 allelic state for genome stability, clinical presentation and outcomes in myelodysplastic syndromes. Nat Med. 2020;26:1549–56. https://doi.org/10.1038/s41591-020-1008-z.
Grob T, Al Hinai ASA, Sanders MA, Kavelaars FG, Rijken M, Gradowska PL, et al. Molecular characterization of mutant TP53 acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood. 2022;139:2347–54. https://doi.org/10.1182/blood.2021014472.
Garcia-Manero G. Myelodysplastic syndromes: 2023 update on diagnosis, risk-stratification, and management. Am J Hematol. 2023;98:1307–25. https://doi.org/10.1002/ajh.26984.
Alessandrino EP, Della Porta MG, Pascutto C, Bacigalupo A, Rambaldi A. Should cytoreductive treatment be performed before transplantation in patients with high-risk myelodysplastic syndrome? J Clin Oncol. 2013;31:2761–2. https://doi.org/10.1200/JCO.2012.48.0525.
Duarte FB, Moura ATG, Funke VAM, Colturato VAR, Hamerschlak N, Vilela NC, et al. Impact of treatment prior to allogeneic transplantation of hematopoietic stem cells in patients with myelodysplastic syndrome: results of the Latin American Bone Marrow Transplant Registry. Biol Blood Marrow Transpl. 2020;26:1021–4. https://doi.org/10.1016/j.bbmt.2020.01.030.
Oran B, Kongtim P, Popat U, de Lima M, Jabbour E, Lu X, et al. Cytogenetics, donor type, and use of hypomethylating agents in myelodysplastic syndrome with allogeneic stem cell transplantation. Biol Blood Marrow Transpl. 2014;20:1618–25. https://doi.org/10.1016/j.bbmt.2014.06.022.
Damaj G, Mohty M, Robin M, Michallet M, Chevallier P, Beguin Y, et al. Upfront allogeneic stem cell transplantation after reduced-intensity/nonmyeloablative conditioning for patients with myelodysplastic syndrome: a study by the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. Biol Blood Marrow Transpl. 2014;20:1349–55. https://doi.org/10.1016/j.bbmt.2014.05.010.
Zhang Y, Liu C, Zhang R, Shi Y, Li X, Yu J, et al. Impact of treatments before allogeneic hematopoietic stem cell transplantation in patients with higher-risk myelodysplastic syndrome. Leuk Res. 2023;124:106997 https://doi.org/10.1016/j.leukres.2022.106997.
Fransolet G, Ehx G, Somja J, Delens L, Hannon M, Muller J, et al. Azacytidine mitigates experimental sclerodermic chronic graft-versus-host disease. J Hematol Oncol. 2016;9:53. https://doi.org/10.1186/s13045-016-0281-2.
Sanchez-Abarca LI, Gutierrez-Cosio S, Santamaria C, Caballero-Velazquez T, Blanco B, Herrero-Sanchez C, et al. Immunomodulatory effect of 5-azacytidine (5-azaC): potential role in the transplantation setting. Blood. 2010;115:107–21. https://doi.org/10.1182/blood-2009-03-210393.
Damaj G, Duhamel A, Robin M, Beguin Y, Michallet M, Mohty M, et al. Impact of azacitidine before allogeneic stem-cell transplantation for myelodysplastic syndromes: a study by the Societe Francaise de Greffe de Moelle et de Therapie-Cellulaire and the Groupe-Francophone des Myelodysplasies. J Clin Oncol. 2012;30:4533–40. https://doi.org/10.1200/JCO.2012.44.3499.
Potter VT, Iacobelli S, van Biezen A, Maertens J, Bourhis JH, Passweg JR, et al. Comparison of intensive chemotherapy and hypomethylating agents before allogeneic stem cell transplantation for advanced myelodysplastic syndromes: a study of the Myelodysplastic Syndrome Subcommittee of the Chronic Malignancies Working Party of the European Society for Blood and Marrow Transplant Research. Biol Blood Marrow Transpl. 2016;22:1615–20. https://doi.org/10.1016/j.bbmt.2016.05.026.
Chen Y, Huang F, Xuan L, Zhang Y, Fan Z, Xu N, et al. Upfront transplantation may have better outcomes than pretransplant cytoreductive therapy for treating patients with MDS-EB-1 or MDS-EB-2. Int J Cancer. 2021;149:1109–20. https://doi.org/10.1002/ijc.33608.
Nawas MT, Kosuri S. Utility or futility? A contemporary approach to allogeneic hematopoietic cell transplantation for TP53-mutated MDS/AML. Blood Adv. 2024;8:553–61. https://doi.org/10.1182/bloodadvances.2023010417.
Wong TN, Ramsingh G, Young AL, Miller CA, Touma W, Welch JS, et al. Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature. 2015;518:552–5. https://doi.org/10.1038/nature13968.
Jacoby MA, Duncavage EJ, Chang GS, Miller CA, Shao J, Elliott K, et al. Subclones dominate at MDS progression following allogeneic hematopoietic cell transplant. JCI Insight. 2018.https://doi.org/10.1172/jci.insight.98962.
Mo XD, Qin YZ, Zhang XH, Xu LP, Wang Y, Yan CH, et al. Minimal residual disease monitoring and preemptive immunotherapy in myelodysplastic syndrome after allogeneic hematopoietic stem cell transplantation. Ann Hematol. 2016;95:1233–40. https://doi.org/10.1007/s00277-016-2706-y.
Ma YY, Wei ZL, Xu YJ, Shi JM, Yi H, Lai YR, et al. Poor pretransplantation minimal residual disease clearance as an independent prognostic risk factor for survival in myelodysplastic syndrome with excess blasts: a multicenter, retrospective cohort study. Cancer. 2023;129:2013–22. https://doi.org/10.1002/cncr.34762.
Tobiasson M, Pandzic T, Illman J, Nilsson L, Westrom S, Ejerblad E, et al. Patient-specific measurable residual disease markers predict outcome in patients with myelodysplastic syndrome and related diseases after hematopoietic stem-cell transplantation. J Clin Oncol. 2024;42:1378–90. https://doi.org/10.1200/JCO.23.01159.
Ciurea SO, Chilkulwar A, Saliba RM, Chen J, Rondon G, Patel KP, et al. Prognostic factors influencing survival after allogeneic transplantation for AML/MDS patients with TP53 mutations. Blood. 2018;131:2989–92. https://doi.org/10.1182/blood-2018-02-832360.
Hientz K, Mohr A, Bhakta-Guha D, Efferth T. The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget. 2017;8:8921–46. https://doi.org/10.18632/oncotarget.13475.
Funding
The study was supported by the National Natural Science Foundation of China (grant numbers: 82070179, 82170210, and 81970158) and the Zhejiang Province Vanguard Goose-Leading Initiative (grant number: 2024SSYS0023).
Author information
Authors and Affiliations
Contributions
SJM, JBQ, and YTT designed the whole study. ZYM, LY, OYGF, YJ, YYS, LJP, LY, LXY, YBD, CY, LLZ, XY, SPF, XHW, HHX, GQY, FHR, SJ, ZWY, HJS, ZY, WWJ, CZ, and WGQ collected data; JBQ, YTT and WXY organized data. JBQ and YTT analyzed and interpreted the data, and wrote the manuscript, which was proofreaded by HH and SJM. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations. Ethical approval was obtained from the Clinical Research Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine (No. IIT20240717B). Informed consent was obtained from each participant or their legal guardians in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41409_2024_2486_MOESM1_ESM.docx
Figure S1. Outcomes after hematopoietic stem cell transplantation of patients with myelodysplastic neoplasms according to different TP53 allelic state.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, B., Yang, T., Zhao, Y. et al. Better pre-transplant treatment options for TP53-mutated MDS: cytoreductive or non-cytoreductive therapy?. Bone Marrow Transplant 60, 326–334 (2025). https://doi.org/10.1038/s41409-024-02486-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41409-024-02486-x