Abstract
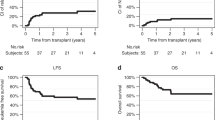
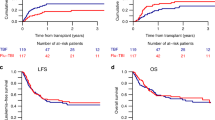
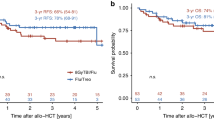
The efficacy of thiotepa, busulfan, and fludarabine (TBF) conditioning in lymphoproliferative disorders remains under investigation. We analyzed outcomes in 157 patients with lymphoid malignancies who underwent a first allogeneic hematopoietic stem cell transplantation (alloHCT) following TBF conditioning. Non-relapse mortality (NRM) at 3 years reached 32%, while the cumulative incidence of relapse (CIR) was 19%. At 3 years, progression-free survival (PFS), overall survival (OS), and graft-versus-host disease (GVHD)-free/relapse-free survival (GRFS) were 49.7%, 58.0%, and 44.2%, respectively. The cumulative incidences of grade III-IV acute GVHD at 100 days and moderate-to-severe chronic GVHD at 3 years were 8.3% and 12%, respectively. On multivariate analysis, patients receiving high-dose thiotepa (10 mg/kg) demonstrated a significantly lower CIR than those receiving low-dose thiotepa (5 mg/kg) (HR 2.95 [95% CI, 1.37–6.33], p = 0.006), with no significant effect on NRM. Female donor-to-male recipient transplants were associated with reduced OS (HR 2.0 [95% CI, 1.17–3.44], p = 0.011) and increased NRM (HR 2.43 [95% CI, 1.29–4.35], p = 0.005). TBF conditioning demonstrated a substantial anti-tumor effect, counterbalanced by elevated toxicity. Careful patient selection and effective toxicity mitigation strategies are essential to ensure individuals can tolerate TBF’s toxicity while maximizing its benefits in disease control.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Snowden JA, Sánchez-Ortega I, Corbacioglu S, Basak GW, Chabannon C, de la Camara R, et al. Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2022. Bone Marrow Transpl. 2022;57:1217–39.
Copelan EA. Hematopoietic Stem-Cell Transplantation. N Engl J Med. 2006;354:1813–26.
Passweg, Baldomero JR, Ciceri H, Corbacioglu F, Cámara S, de la R, et al. Hematopoietic cell transplantation and cellular therapies in Europe 2021. The second year of the SARS-CoV-2 pandemic. A Report from the EBMT Activity Survey. Bone Marrow Transpl. 2023;58:647.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2009;15:1628–33.
Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2009;15:367–9.
Spyridonidis A, Labopin M, Savani BN, Niittyvuopio R, Blaise D, Craddock C, et al. Redefining and measuring transplant conditioning intensity in current era: a study in acute myeloid leukemia patients. Bone Marrow Transpl. 2020;55:1114–25.
Gyurkocza B, Sandmaier BM. Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood. 2014;124:344–53.
Saraceni F, Beohou E, Labopin M, Arcese W, Bonifazi F, Stepensky P, et al. Thiotepa, busulfan and fludarabine compared to busulfan and cyclophosphamide as conditioning regimen for allogeneic stem cell transplant from matched siblings and unrelated donors for acute myeloid leukemia. Am J Hematol. 2018;93:1211–9.
Sanz J, Boluda JCH, Martín C, González M, Ferrá C, Serrano D, et al. Single-unit umbilical cord blood transplantation from unrelated donors in patients with hematological malignancy using busulfan, thiotepa, fludarabine and ATG as myeloablative conditioning regimen. Bone Marrow Transpl. 2012;47:1287–93.
Raiola AM, Dominietto A, Ghiso A, Grazia CD, Lamparelli T, Gualandi F, et al. Unmanipulated Haploidentical Bone Marrow Transplantation and Posttransplantation Cyclophosphamide for Hematologic Malignancies after Myeloablative Conditioning. Biol Blood Marrow Transpl. 2013;19:117–22.
Esquirol A, Pascual MJ, Ortiz M, Piñana JL, Ferra C, Garcia Cadenas I, et al. Single-agent GvHD prophylaxis with tacrolimus after post-transplant high-dose cyclophosphamide is a valid option for haploidentical transplantation in adults with hematological malignancies. Bone Marrow Transpl. 2017;52:1273–9.
Pagliardini T, Castagna L, Harbi S, Porta MD, Rey J, Fürst S, et al. Thiotepa, Fludarabine, and Busulfan Conditioning Regimen before T Cell–Replete Haploidentical Transplantation with Post-Transplant Cyclophosphamide for Acute Myeloid Leukemia: A Bicentric Experience of 100 Patients. Biol Blood Marrow Transpl. 2019;25:1803–9.
Duléry R, Bastos J, Paviglianiti A, Malard F, Brissot E, Battipaglia G, et al. Thiotepa, Busulfan, and Fludarabine Conditioning Regimen in T Cell-Replete HLA-Haploidentical Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transpl. 2019;25:1407–15.
Shouval R, Vega Y, Fein JA, Danylesko I, Shem Tov N, Yerushalmi R, et al. Allogeneic hematopoietic stem cell transplantation with fludarabine, busulfan, and thiotepa conditioning is associated with favorable outcomes in myelofibrosis. Bone Marrow Transpl. 2020;55:147–56.
Memoli M, Paviglianiti A, Malard F, Battipaglia G, Brissot E, Médiavilla C, et al. Thiotepa-busulfan-fludarabine as a conditioning regimen for patients with myelofibrosis undergoing allogeneic hematopoietic transplantation: a single center experience. Leuk Lymphoma. 2021;62:419–27.
Holtan SG, DeFor TE, Lazaryan A, Bejanyan N, Arora M, Brunstein CG, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125:1333–8.
Iacobelli S. EBMT Statistical Committee. Suggestions on the use of statistical methodologies in studies of the European Group for Blood and Marrow Transplantation. Bone Marrow Transpl. 2013;48:S1–37.
Ruggeri A, Labopin M, Ciceri F, Mohty M, Nagler A. Definition of GvHD-free, relapse-free survival for registry-based studies: an ALWP–EBMT analysis on patients with AML in remission. Bone Marrow Transpl. 2016;51:610–1.
Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. J Am Stat Assoc. 1958;53:457–81.
Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann Stat. 1988;16:1141–54.
Andersen PK, Klein JP, Zhang MJ. Testing for centre effects in multi-centre survival studies: a Monte Carlo comparison of fixed and random effects tests. Stat Med. 1999;18:1489–500.
Bacigalupo A, Raiola AM, Lamparelli T, Gualandi F, Occhini D, Bregante S, et al. Thiotepa-based reduced intensity conditioning regimen: a 10 year follow up. Bone Marrow Transpl. 2007;40:1091–3.
Fox ML, García-Cadenas I, Pérez AM, Villacampa G, Piñana JL, Ortí G, et al. Feasibility of thiotepa addition to the fludarabine-busulfan conditioning with tacrolimus/sirolimus as graft vs host disease prophylaxis. Leuk Lymphoma. 2020;61:1823–32.
Saraceni F, Labopin M, Hamladji RM, Mufti G, Socié G, Shimoni A, et al. Thiotepa-busulfan-fludarabine compared to busulfan-fludarabine for sibling and unrelated donor transplant in acute myeloid leukemia in first remission. Oncotarget. 2018;9:3379–93.
El-Cheikh J, Massoud R, Moukalled N, Haffar B, Assi H, Zahreddine A, et al. Thiotepa 10 mg/kg Treatment Regimen Is Superior to Thiotepa 5 mg/kg in TBF Conditioning in Patients Undergoing Allogeneic Stem-Cell Transplantation. Clin Lymphoma Myeloma Leuk. 2018;18:368–74.
El-Cheikh J, Labopin M, Al-Chami F, Bazarbachi A, Angelucci E, Santarone S, et al. Effect of the Thiotepa Dose in the TBF Conditioning Regimen in Patients Undergoing Allogeneic Stem Cell Transplantation for Acute Myeloid Leukemia in Complete Remission: A Report From the EBMT Acute Leukemia Working Party. Clin Lymphoma Myeloma Leuk. 2020;20:296–304.
Robinson SP, Boumendil A, Finel H, Blaise D, Poiré X, Nicolas-Virelizier E, et al. Autologous stem cell transplantation for relapsed/refractory diffuse large B-cell lymphoma: efficacy in the rituximab era and comparison to first allogeneic transplants. A report from the EBMT Lymphoma Working Party. Bone Marrow Transpl. 2016;51:365–71.
Bacher U, Klyuchnikov E, Le-Rademacher J, Carreras J, Armand P, Bishop MR, et al. Conditioning regimens for allotransplants for diffuse large B-cell lymphoma: myeloablative or reduced intensity? Blood. 2012;120:4256–62.
Ghosh N, Ahmed S, Ahn KW, Khanal M, Litovich C, Aljurf M, et al. Association of Reduced-Intensity Conditioning Regimens With Overall Survival Among Patients With Non-Hodgkin Lymphoma Undergoing Allogeneic Transplant. JAMA Oncol. 2020;6:1011–8.
Nakasone H, Remberger M, Tian L, Brodin P, Sahaf B, Wu F, et al. Risks and benefits of sex-mismatched hematopoietic cell transplantation differ according to conditioning strategy. Haematologica. 2015;100:1477–85.
Gratwohl A, Hermans J, Niederwieser D, van Biezen A, van Houwelingen HC, Apperley J, et al. Female donors influence transplant-related mortality and relapse incidence in male recipients of sibling blood and marrow transplants. Hematol J J Eur Haematol Assoc. 2001;2:363–70.
Ijaz A, Khan AY, Malik SU, Faridi W, Fraz MA, Usman M, et al. Significant Risk of Graft-versus-Host Disease with Exposure to Checkpoint Inhibitors Before and After Allogeneic transplantation. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2018;25:94.
Haverkos BM, Abbott D, Hamadani M, Armand P, Flowers ME, Merryman R, et al. PD-1 blockade for relapsed lymphoma post–allogeneic hematopoietic cell transplant: high response rate but frequent GVHD. Blood. 2017;130:221.
Acknowledgements
We thank the patients, as well as the nursing and support staff at the Hematopoietic Cell Transplant and Cell Therapy Programs from the 10 collaborating institutions. We also thank the GETH-TC group for its support, particularly Ángel Cedillo and Silvia Filaferro for their invaluable contribution in collecting the clinical data for this manuscript.
Author information
Authors and Affiliations
Contributions
Contribution: MP and AM designed the study. DFM and AP performed the statistical analysis. MP, DFM and AP created the figures and wrote the manuscript. AM, AB, JS, MJP, BH, CS, AB, MQS, MR, AN, IE, MH, LB and AJS contributed to patient care and data collection, provided valuable input during the study, and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Written informed consent was obtained from all study subjects.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peña, M., Martinez, D.F., Paviglianiti, A. et al. Thiotepa, busulfan and fludarabine conditioning regimen for adult patients with lymphoid malignancies undergoing allogeneic stem cell transplantation: a study on behalf of GETH-TC. Bone Marrow Transplant 60, 795–803 (2025). https://doi.org/10.1038/s41409-025-02559-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41409-025-02559-5