Abstract
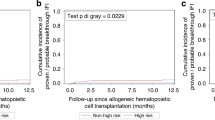
With the aim to reduce the incidence of invasive fungal infections (IFI) after allogeneic hematopoietic stem cell transplantation (allo-HSCT), the ECIL group recommends the use of drugs active against molds such as posaconazole instead of fluconazole in high-risk (HR) IFI patients. But data to support this recommendation are poor. The aim of this monocentric study was to compare retrospectively the use of fluconazole (n = 96) vs. posaconazole (n = 63), as primary antifungal prophylaxis within the first 90 days (D) post-transplant in a cohort of patients at HR-IFI (n = 159). HR-IFI was defined by the use of an alternative donor, post-transplant cyclophosphamide and/or sequential conditioning regimen, and/or an active disease at transplant or a previous allo-HSCT. Incidences of D90, 6-month, 1-year and 2-year CI of IFI as well as D90 primary prophylaxis failure (IFI resulting in the initiation of a curative antifungal therapy or a permanent discontinuation of the prophylaxis for toxicity) were similar between both groups. However, the number of probable/proven IFI that occurred between D0 and D90 was the double in the fluco group (9 vs. 4). Also, no proven IFI (vs. 4) or mucormycoses (vs. 1) or IFI related death (vs. 4) occurred in the posa group in the first 90 days. Posaconazole thus appears to be a good option to prevent IFI after allo-HSCT in patients at HR-IFI.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Individual data used to generate these results are available upon personal request. Please contact the corresponding author.
References
Döhner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140:1345–77.
Gökbuget N, Boissel N, Chiaretti S, Dombret H, Doubek M, Fielding A, et al. Management of ALL in adults: 2024 ELN recommendations from a European expert panel. Blood 2024;143:1903–30.
Papanicolaou GA, Chen M, He N, Martens MJ, Kim S, Batista MV, et al. Incidence and impact of fungal infections in post-transplantation cyclophosphamide-based graft-versus-host disease prophylaxis and haploidentical hematopoietic cell transplantation: a Center for International Blood and Marrow Transplant Research 4. Transpl Cell Ther. 2024;30:114.
Goodman JL, Winston DJ, Greenfield RA, Chandrasekar PH, Fox B, Kaizer H, et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med. 1992;326:845–51.
Slavin MA, Osborne B, Adams R, Levenstein MJ, Schoch HG, Feldman AR, et al. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation-a prospective, randomized, double-blind study. J Infect Dis. 1995;171:1545–52.
Marr KA, Seidel K, Slavin MA, Bowden RA, Schoch HG, Flowers ME, et al. Prolonged fluconazole prophylaxis is associated with persistent protection against candidiasis-related death in allogeneic marrow transplant recipients: long-term follow-up of a randomized, placebo-controlled trial. Blood. 2000;96:2055–261.
Girmenia C, Raiola AM, Piciocchi A, Algarotti A, Stanzani M, Cudillo L, et al. Incidence and outcome of invasive fungal diseases after allogeneic stem cell transplantation: a prospective study of the Gruppo Italiano Trapianto Midollo Osseo (GITMO). Biol Blood Marrow Transpl. 2014;20:872–80.
Maertens JA, Girmenia C, Brüggemann RJ, Duarte RF, Kibbler CC, Ljungman P, et al. European Conference on Infections in Leukaemia (ECIL), a joint venture of the European Group for Blood and Marrow Transplantation (EBMT), the European Organization for Research and Treatment of Cancer (EORTC), the Immunocompromised Host Society (ICHS) and European Conference on Infections in Leukaemia (ECIL), a joint venture of the European Group for Blood and Marrow Transplantation (EBMT), the European Organization for Research and Treatment of Cancer (EORTC), the Immunocompromised Host Society (ICHS) and the European LeukemiaNet (ELN). European guidelines for primary antifungal prophylaxis in adult haematology patients: summary of the updated recommendations from the European Conference on Infections in Leukaemia. J Antimicrob Chemother. 2018;73:3221–30.
Patterson TF, Thompson GR 3rd, Denning DW, Fishman JA, Hadley S, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63:e1–e60.
Stemler J, Mellinghoff SC, Khodamoradi Y, Sprute R, Classen AY, Zapke SE, et al. Primary prophylaxis of invasive fungal diseases in patients with haematological malignancies: 2022 update of the recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society for Haematology and Medical Oncology (DGHO). J Antimicrob Chemother. 2023;78:1813–26.
Lewalle P, Pochon C, Michallet M, Turlure P, Brissot E, Paillard C. et al. Prophylaxie des infections post-allogreffe : recommandations de la Société francophone de greffe de moelle et de thérapie cellulaire (SFGM-TC) [Prophylaxis of infections post-allogeneic transplantation: Guidelines from the Francophone Society of Bone Marrow Transplantation and Cellular Therapy (SFGM-TC)]. Bull Cancer. 2019;106:S23–S34.
Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356:335–47.
Wingard JR, Carter SL, Walsh TJ, Kurtzberg J, Small TN, Baden LR, et al. Blood and Marrow Transplant Clinical Trials Network. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood. 2010;116:5111–8.
Marr KA, Crippa F, Leisenring W, Hoyle M, Boeckh M, Balajee SA, et al. Itraconazole versus fluconazole for prevention of fungal infections in patients receiving allogeneic stem cell transplants. Blood. 2004;103:1527–33.
Marks DI, Pagliuca A, Kibbler CC, Glasmacher A, Heussel CP, Kantecki M, et al. Voriconazole versus itraconazole for antifungal prophylaxis following allogeneic haematopoietic stem-cell transplantation. Br J Haematol. 2011;155:318–27.
Dvorak CC, Fisher BT, Esbenshade AJ, Nieder ML, Alexander S, Steinbach WJ, et al. A randomized trial of caspofungin vs triazoles prophylaxis for invasive fungal disease in pediatric allogeneic hematopoietic cell transplant. J Pediatr Infect Dis Soc. 2020;10:417–25.
Koh LP, Kurup A, Goh YT, Fook-Chong SMC, Tan PHC. Randomized trial of fluconazole versus low-dose amphotericin B in prophylaxis against fungal infections in patients undergoing hematopoietic stem cell transplantation. Am J Hematol. 2002;71:260–7.
Oren I, Rowe JM, Sprecher H, Tamir A, Benyamini N, Akria L, et al. A prospective randomized trial of itraconazole vs fluconazole for the prevention of fungal infections in patients with acute leukemia and hematopoietic stem cell transplant recipients. Bone Marrow Transpl. 2006;38:127–34.
Park S, Kim K, Jang JH, Kim SJ, Kim WS, Chung DR, et al. Randomized trial of micafungin versus fluconazole as prophylaxis against invasive fungal infections in hematopoietic stem cell transplant recipients. J Infect. 2016;73:496–505.
Sun Y, Hu J, Huang H, Chen J, Li J, Ma J, et al. Fluconazole is as effective as other anti-mold agents in preventing early invasive fungal disease after allogeneic stem cell transplantation: assessment of antifungal therapy in haematological disease in China. Transl Cancer Res. 2020;9:6900–11.
Wang CH, Kan LP, Lin HA, Chang FY, Wang NC, Lin TY, et al. Clinical efficacy and safety of primary antifungal prophylaxis with posaconazole versus fluconazole in allogeneic blood hematopoietic stem cell transplantation recipients—A retrospective analysis of a single medical center in Taiwan. J Microbiol Immunol Infect. 2016;49:531–8.
Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356:348–59.
-Haute Autorité de Santé [Internet]. [cited 2025 Mar 17]. POSACONAZOLE EG (posaconazole). Availablefrom: https://www.hassante.fr/jcms/p_3146433/fr/posaconazole-eg-posaconazole.
Cornely OA, Duarte RF, Haider S, Chandrasekar P, Helfgott D, Jiménez JL, et al. Phase 3 pharmacokinetics and safety study of a posaconazole tablet formulation in patients at risk for invasive fungal disease. J Antimicrob Chemother. 2016;71:718–26.
Cornely OA, Robertson MN, Haider S, Grigg A, Geddes M, Aoun M, et al. Pharmacokinetics and safety results from the Phase 3 randomized, open-label, study of intravenous posaconazole in patients at risk of invasive fungal disease. J Antimicrob Chemother. 2017;72:3406–13.
Oh J, Kang CI, Kim SH, Huh K, Cho SY, Chung DR, et al. Antifungal prophylaxis with posaconazole tablet and oral suspension in patients with haematologic malignancy: Therapeutic drug monitoring, efficacy and risk factors for the suboptimal level. Mycoses. 2020;63:89–94.
Peterlin P, Chauvin C, Le Gouill S, Pere M, Dalichampt M, Guillaume T, et al. Fungal prophylaxis with a gastro-resistant posaconazole tablet for patients with hematological malignancies in the POSANANTES study. Antimicrob Agents Chemother. 2018;62:e01746–e01817.
Miceli MH, Perissinotti AJ, Kauffman CA, Couriel DR. Serum posaconazole levels among haematological cancer patients taking extended release tablets is affected by body weight and diarrhoea: single centre retrospective analysis. Mycoses. 2015;58:432–6.
Marty FM, Ljungman P, Chemaly RF, Maertens J, Dadwal SS, Duarte RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med. 2017;377:2433–44.
Marshall WL, McCrea JB, Macha S, Menzel K, Liu F, van Schanke A, et al. Pharmacokinetics and tolerability of letermovir coadministered with azole antifungals (posaconazole or voriconazole) in healthy subjects. J Clin Pharm. 2018;58:897–904.
Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, et al. Revision and update of consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2020;71:1367–76.
Dekkers BGJ, Bakker M, van der Elst KCM, Sturkenboom MGG, Veringa A, Span LFR, et al. Therapeutic drug monitoring of posaconazole: an update. Curr Fungal Infect Rep. 2016;10:51–61.
Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the mount sinai acute GVHD International Consortium. Biol Blood Marrow Transpl. 2016;22:4–10.
Martin PJ, Lee SJ, Przepiorka D, Horowitz MM, Koreth J, Vogelsang GB, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: VI. the 2014 clinical trial design working group report. Biol Blood Marrow Transpl. 2015;21:1343–59.
Bayraktar UD, Shpall EJ, Liu P, Ciurea SO, Rondon G, de Lima M, et al. Hematopoietic cell transplantation–specific comorbidity index predicts inpatient mortality and survival in patients who received allogeneic transplantation admitted to the intensive care unit. J Clin Oncol. 2013;31:4207–14.
Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the disease risk index for allogeneic stem cell transplantation. Blood. 2014;123:3664–71.
Campen CJ, Vogel WH, Shah PJ. Managing drug interactions in cancer therapy: a guide for the advanced practitioner. J Adv Pr Oncol. 2017;8:609–20.
Marr KA, Leisenring W, Crippa F, Slattery JT, Corey L, Boeckh M, et al. Cyclophosphamide metabolism is affected by azole antifungals. Blood. 2004;103:1557–9.
Neofytos D, Horn D, Anaissie E, Steinbach W, Olyaei A, Fishman J, et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of multicenter prospective antifungal therapy (PATH) alliance registry. Clin Infect Dis. 2009;48:265–73.
Hoenigl M, Sprute R, Egger M, Arastehfar A, Cornely OA, Krause R, et al. The antifungal pipeline: fosmanogepix, ibrexafungerp, olorofim, opelconazole, and rezafungin. Drugs. 2021;81:1703–29.
Acknowledgements
The authors wish to thank all investigators and data managers for their dedicated patient care. Medical writing for this manuscript was assisted by MPIYP (MC Béné), Paris, France.
Author information
Authors and Affiliations
Contributions
A.L.B and V.L designed, performed, coordinated the research, analyzed, interpreted the data, and wrote the manuscript. M.J performed the statistical analyses. A.G, P.P, S.V, A-M.F, T.G, P.C contributed data and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics declarations
All methods were performed in accordance with the relevant guidelines and regulations. Ethics committee was not requested for this study because the design was a retrospective comparison of two antifungal prophylaxis strategies for allo-HSCT recipients at two different periods of time. No experimental drug was given and antifungal strategies were supported by ECIL guidelines that have evolved over the years. However, all patients provided informed consent for collection of their personal data.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Letailleur, V., Jullien, M., Garnier, A. et al. Posaconazole versus fluconazole as primary antifungal prophylaxis for patients at high risk of invasive fungal infections receiving allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 60, 1092–1101 (2025). https://doi.org/10.1038/s41409-025-02589-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41409-025-02589-z