Abstract
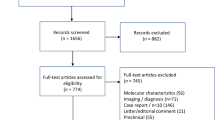
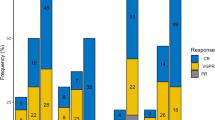
Chimeric antigen receptor (CAR) T cell therapy is effective for multiple myeloma (MM), yet patients with plasmacytomas, either paraskeletal disease (PSD) or extramedullary disease (EMD), have poorer outcomes. To better distinguish the effects of EMD and PSD on outcomes, we conducted a single-center, retrospective study of 134 relapsed/refractory MM patients treated with CAR T cells. With a median follow-up of 30.2 months, patients with EMD (n = 34) had significantly worse progression-free survival (PFS) and overall survival (OS) than those with bone marrow-only disease (n = 75): PFS was 24.2 months vs. 9.0 months (HR 2.15, 95% CI 1.31–3.54), and OS was not reached vs. 24.0 months (HR 3.79, 95% CI 1.81–7.94). In contrast, patients with PSD did not experience significantly shorter PFS or OS. Among patients with EMD, those with high extramedullary tumor burden had a lower overall response rate (ORR). For extramedullary tumor burden <25 cm2, 25–50 cm2, and >50 cm2, ORR was 81.0% (66.7% complete response, CR), 83.3% (50% CR), and 57.1% (0% CR), respectively. Importantly, EMD-positive relapses post-CAR T cell therapy comprised half of all relapses observed. In summary, our findings indicate that EMD, but not PSD, is strongly associated with poor outcomes with CAR T cell therapy.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data generated or analyzed for the current study are available from the corresponding author on reasonable request.
References
Munshi NC, Anderson LD Jr, Shah N, Madduri D, Berdeja J, Lonial S, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384:705–16.
Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398:314–24.
Bansal R, Rakshit S, Kumar S. Extramedullary disease in multiple myeloma. Blood Cancer J. 2021;11:161.
Bladé J, Beksac M, Caers J, Jurczyszyn A, von Lilienfeld-Toal M, Moreau P, et al. Extramedullary disease in multiple myeloma: a systematic literature review. Blood Cancer J. 2022;12:45.
Weinstock M, Ghobrial IM. Extramedullary multiple myeloma. Leuk lymphoma. 2013;54:1135–41.
Rosiñol L, Beksac M, Zamagni E, Van de Donk NW, Anderson KC, Badros A, et al. Expert review on soft‐tissue plasmacytomas in multiple myeloma: definition, disease assessment and treatment considerations. Br J Haematol. 2021;194:496–507.
Pour L, Sevcikova S, Greslikova H, Kupska R, Majkova P, Zahradova L, et al. Soft-tissue extramedullary multiple myeloma prognosis is significantly worse in comparison to bone-related extramedullary relapse. Haematologica. 2014;99:360.
Batsukh K, Lee S-E, Min GJ, Park SS, Jeon Y-W, Yoon J-H, et al. Distinct clinical outcomes between paramedullary and extramedullary lesions in newly diagnosed multiple myeloma. Immune Netw. 2017;17:250.
Hashmi H, Hansen DK, Peres LC, Puglianini OC, Freeman C, De Avila G, et al. Factors associated with refractoriness or early progression after idecabtagene vicleucel in patients with relapsed/refractory multiple myeloma: US Myeloma Immunotherapy Consortium real world experience. Haematologica. 2024;109:1514.
San-Miguel J, Dhakal B, Yong K, Spencer A, Anguille S, Mateos M-V, et al. Cilta-cel or standard care in lenalidomide-refractory multiple myeloma. N Engl J Med. 2023;389:335–47.
Rodriguez-Otero P, Ailawadhi S, Arnulf B, Patel K, Cavo M, Nooka AK, et al. Ide-cel or standard regimens in relapsed and refractory multiple myeloma. N Engl J Med. 2023;388:1002–14.
Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–e46.
Lin Y, Raje NS, Berdeja JG, Siegel DS, Jagannath S, Madduri D, et al. Idecabtagene vicleucel for relapsed and refractory multiple myeloma: post hoc 18-month follow-up of a phase 1 trial. Nat Med. 2023;29:2286–94.
Zanwar S, Sidana S, Shune L, Puglianini OC, Pasvolsky O, Gonzalez R, et al. Impact of extramedullary multiple myeloma on outcomes with idecabtagene vicleucel. J Hematol Oncol. 2024;17:42.
Martin T, Usmani SZ, Berdeja JG, Agha M, Cohen AD, Hari P, et al. Ciltacabtagene autoleucel, an anti–B-cell maturation antigen chimeric antigen receptor T-cell therapy, for relapsed/refractory multiple myeloma: CARTITUDE-1 2-year follow-up. J Clin Oncol. 2023;41:1265–74.
Dima D, Abdallah A-O, Davis JA, Awada H, Goel U, Rashid A, et al. Impact of extraosseous extramedullary disease on outcomes of patients with relapsed-refractory multiple myeloma receiving standard-of-care chimeric antigen receptor T-cell therapy. Blood Cancer J. 2024;14:90.
Zugasti I, Tormo-Ratera M, Oliver-Caldes A, Soler-Perromat JC, Gonzalez-Calle V, Moreno DF, et al. Clinical impact of [18F] FDG-PET/CT in ARI0002h treatment, a CAR-T against BCMA for relapsed/refractory multiple myeloma. Blood Adv. 2025;9:571–82 .
Billecke L, Murga Penas EM, May AM, Engelhardt M, Nagler A, Leiba M, et al. Cytogenetics of extramedullary manifestations in multiple myeloma. Br J Haematol. 2013;161:87–94.
Besse L, Sedlarikova L, Greslikova H, Kupska R, Almasi M, Penka M, et al. Cytogenetics in multiple myeloma patients progressing into extramedullary disease. Eur J Haematol. 2016;97:93–100.
Avivi I, Cohen YC, Suska A, Shragai T, Mikala G, Garderet L, et al. Hematogenous extramedullary relapse in multiple myeloma‐a multicenter retrospective study in 127 patients. Am J Hematol. 2019;94:1132–40.
Dahl IMS, Rasmussen T, Kauric G, Husebekk A. Differential expression of CD56 and CD44 in the evolution of extramedullary myeloma. Br J Haematol. 2002;116:273–7.
Kremer M, Ott G, Nathrath M, Specht K, Stecker K, Alexiou C, et al. Primary extramedullary plasmacytoma and multiple myeloma: phenotypic differences revealed by immunohistochemical analysis. J Pathol: A J Pathol Soc Gt Br Irel. 2005;205:92–101.
John M, Helal M, Duell J, Mattavelli G, Stanojkovska E, Afrin N, et al. Spatial transcriptomics reveals profound subclonal heterogeneity and T-cell dysfunction in extramedullary myeloma. Blood J. 2024;144:2121–35.
Acknowledgements
The authors extend their gratitude to their patients and caregivers who made this work possible.
Author information
Authors and Affiliations
Contributions
DP conceptualized the study, collected and analyzed data and wrote the manuscript. SR conceptualized the study, analyzed and interpreted data, and edited the manuscript. THM collected data and edited the manuscript. TS, WF, and EM analyzed and interpreted data and edited the manuscript. JR, SP, SJ, ACR, LJS, ST, CS, and HJC reviewed and edited the final manuscript.
Corresponding author
Ethics declarations
Competing interests
DP: Honoraria from Sanofi. THM: Advisory board fees for Sanofi. JR: Consultant/advisory board: Janssen, BMS, Pfizer, Karyopharm, Sanofi, Takeda, Genentech, Abbvie, Regeneron, Forus, Menarini, Speakers Bureau: Janssen, BMS, Sanofi, Adaptive Biotechnologies. SP: Advisory board for Grail; research support from Celgene/BMS Corporation, Grail, Caribou. SJ: Consulting for Janssen Pharmaceuticals, BMS, Caribou Biosciences, Legend Biotech, Regeneron Pharmaceuticals, Takeda Pharmaceuticals, Sanofi, Poseida Therapeutics; Advisory board for Janssen Pharmaceuticals, BMS, GSK; Data Safety Monitoring Board for Janssen Pharmaceuticals, Sanofi, Genmab. LJS: Consulting/advisory board for Janssen Pharmaceuticals. CR: Advisory board for Janssen Pharmaceuticals, Takeda Pharmaceuticals, BMS, Amgen, Karyopharm Therapeutics. ACR: Consulting for Johnson & Johnson, Adaptive, BMS, Sanofi. HJC: Employment: The Multiple Myeloma Research Foundation, Research funding: Genentech/Roche, BMS, Takeda. The remaining authors declare no relevant competing financial interests.
Ethics approval
Patient data was retrospectively collected and analyzed in accordance with the Declaration of Helsinki. As many patients were deceased, obtaining informed consent was not possible. Therefore, a waiver of informed consent was granted, and the study was approved by the institutional review board of Mount Sinai (IRB#11-01978-MOD0013).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pan, D., Mouhieddine, T.H., Sheng, T. et al. Extramedullary disease but not paraskeletal disease portends inferior outcomes after CAR T cell therapy in multiple myeloma. Bone Marrow Transplant 60, 1114–1119 (2025). https://doi.org/10.1038/s41409-025-02593-3
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41409-025-02593-3
This article is cited by
-
Therapeutic options for extramedullary involvement in multiple myeloma
Clinical and Experimental Medicine (2025)
-
Activity of CAR-T cells and bispecific antibodies in multiple myeloma with extramedullary involvement
Blood Cancer Journal (2025)