Abstract
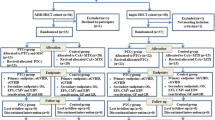
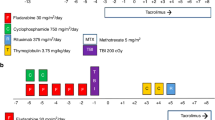
The use of post-transplant cyclophosphamide (PTCy) for graft-versus-host disease (GVHD) prophylaxis in severe aplastic anemia (SAA) remains understudied, particularly beyond haploidentical transplants. We analyzed outcomes of SAA patients who underwent stem cell transplantation (SCT) with PTCy from haploidentical donors (n = 209), HLA-matched sibling donors (MSD, n = 70), and unrelated donors (UD, n = 69) using EBMT data from 2010 to 2022. Median age was 22 years, and median time to transplantation was 8.6 months. For haploidentical, MSD, and UD cohorts, the 100-day cumulative incidence of grade II-IV acute GVHD was 19%, 11%, and 14% (p = 0.15), while grade III-IV was 6%, 3%, and 2% (p = 0.1). Two-year chronic and extensive chronic GVHD were 14%, 13%, and 14% (p = 0.1) and 5%, 6%, and 2% (p = 0.5), respectively. Non-relapse mortality at two years was 24% for haploidentical, 7% for MSD, and 10% for UD (p = 0.003). Two-year overall survival (OS) and GVHD- and relapse-free survival were 66% and 54% for haploidentical, 92% and 70% for MSD, and 81% and 66% for UD (p < 0.001, p = 0.06). In multivariable analysis, MSD and UD were associated with superior OS and GRFS compared to haploidentical. PTCy is safe and effective in SAA patients, though haploidentical SCT had higher NRM, leading to lower survival.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
All data can be obtained from corresponding author for appropriated reasons.
References
Georges GE, Doney K, Storb R. Severe aplastic anemia: allogeneic bone marrow transplantation as first-line treatment. Blood Adv. 2018;2:2020–8.
Young NS. Aplastic anemia. N Engl J Med. 2018;379:1643–56.
Bacigalupo A. How I treat acquired aplastic anemia. Blood. 2016;129:1428–36.
Montoro J, Eikema D-J, Tuffnell J, Potter V, Kalwak K, Halkes CJ et al. Alternative donor transplantation for severe aplastic anemia: a comparative study of the SAAWP EBMT. Blood 2024. https://doi.org/10.1182/blood.2024024173.
Dufour C, Veys P, Carraro E, Bhatnagar N, Pillon M, Wynn R, et al. Similar outcome of upfront‐unrelated and matched sibling stem cell transplantation in idiopathic paediatric aplastic anaemia. A study on behalf of the UK Paediatric BMT Working Party, Paediatric Diseases Working Party and Severe Aplastic Anaemia Working Party of EBMT. Brit J Haematol. 2015;171:585–94.
DeZern AE, Zahurak ML, Symons HJ, Cooke KR, Rosner GL, Gladstone DE, et al. Haploidentical BMT for severe aplastic anemia with intensive GVHD prophylaxis including posttransplant cyclophosphamide. Blood Adv. 2020;4:1770–9.
DeZern AE, Eapen M, Wu J, Talano J-A, Solh M, Saldaña BJD, et al. Haploidentical bone marrow transplantation in patients with relapsed or refractory severe aplastic anaemia in the USA (BMT CTN 1502): a multicentre, single-arm, phase 2 trial. Lancet Haematol. 2022;9:e660–9.
DeZern AE, Zahurak M, Symons H, Cooke K, Jones RJ, Brodsky RA. Alternative donor transplantation with high-dose post-transplantation cyclophosphamide for refractory severe aplastic anemia. Biol Blood Marrow Tr. 2017;23:498–504.
Latour, de RP, Purtill D, Ruggeri A, Sanz G, Michel G, et al. Influence of nucleated cell dose on overall survival of unrelated cord blood transplantation for patients with severe acquired aplastic anemia: a study by Eurocord and the Aplastic Anemia Working party of the European Group for Blood and Marrow Transplantation. Biol Blood Marrow Tr. 2011;17:78–85.
Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transpl. 2008;14:641–50.
McCurdy SR, Kanakry JA, Showel MM, Tsai H-L, Bolaños-Meade J, Rosner GL, et al. Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood. 2015;125:3024–31.
Kasamon YL, Luznik L, Leffell MS, Kowalski J, Tsai H-L, Bolaños-Meade J, et al. Nonmyeloablative HLA-Haploidentical bone marrow transplantation with high-dose posttransplantation cyclophosphamide: effect of HLA disparity on outcome. Biol Blood Marrow Transpl. 2010;16:482–9.
Montoro J, Piñana JL, Hernández-Boluda JC, Hernani R, Lorenzo I, Pérez A, et al. Uniform graft-versus-host disease prophylaxis with posttransplant cyclophosphamide, sirolimus, and mycophenolate mofetil following hematopoietic stem cell transplantation from haploidentical, matched sibling and unrelated donors. Bone Marrow Transpl. 2020;55:2147–59.
Kwon M, (GETH) on behalf of GE de TH y TC, Bailén R, Pascual-Cascón MJ, Gallardo-Morillo AI, Sola AG, et al. Posttransplant cyclophosphamide vs cyclosporin A and methotrexate as GVHD prophylaxis in matched sibling transplantation. Blood Adv. 2019;3:3351–9.
Mielcarek M, Furlong T, O’Donnell PV, Storer BE, McCune JS, Storb R, et al. Posttransplantation cyclophosphamide for prevention of graft-versus-host disease after HLA-matched mobilized blood cell transplantation. Blood. 2016;127:1502–8.
Ruggeri A, Labopin M, Bacigalupo A, Afanasyev B, Cornelissen JJ, Elmaagacli A, et al. Post-transplant cyclophosphamide for graft-versus-host disease prophylaxis in HLA matched sibling or matched unrelated donor transplant for patients with acute leukemia, on behalf of ALWP-EBMT. J Hematol Oncol. 2018;11:40.
Sanz J, Galimard J-E, Labopin M, Afanasyev B, Angelucci E, Ciceri F, et al. Post-transplant cyclophosphamide after matched sibling, unrelated and haploidentical donor transplants in patients with acute myeloid leukemia: a comparative study of the ALWP EBMT. J Hematol Oncol. 2020;13:46.
Dreger P, Sureda A, Ahn KW, Eapen M, Litovich C, Finel H, et al. PTCy-based haploidentical vs matched related or unrelated donor reduced-intensity conditioning transplant for DLBCL. Blood Adv. 2019;3:360–9.
Bolaños-Meade J, Hamadani M, Wu J, Malki MMA, Martens MJ, Runaas L, et al. Post-transplantation cyclophosphamide-based graft-versus-host disease prophylaxis. N Engl J Med. 2023;388:2338–48.
Bailén R, Kwon M, Pascual-Cascón MJ, Ferrà C, Sanz J, Gallardo-Morillo A, et al. Post-transplant cyclophosphamide for GVHD prophylaxis compared to ATG-based prophylaxis in unrelated donor transplantation. Ann Hematol. 2021;100:541–53.
Baron F, Labopin M, Tischer J, Ciceri F, Raiola AM, Blaise D, et al. Comparison of HLA-mismatched unrelated donor transplantation with post-transplant cyclophosphamide versus HLA-haploidentical transplantation in patients with active acute myeloid leukemia. Bone Marrow Transpl. 2022;57:1657–63.
Clay J, Kulasekararaj AG, Potter V, Grimaldi F, McLornan D, Raj K, et al. Nonmyeloablative peripheral blood haploidentical stem cell transplantation for refractory severe aplastic anemia. Biol Blood Marrow Tr. 2014;20:1711–6.
Esteves I, Bonfim C, Pasquini R, Funke V, Pereira NF, Rocha V, et al. Haploidentical BMT and post-transplant Cy for severe aplastic anemia: a multicenter retrospective study. Bone Marrow Transpl. 2015;50:685–9.
Wu L, Zhou M, Li Y, Chen X, Mo W, Wang C et al. Prospective study of a modified post-transplantation cyclophosphamide regimen for severe aplastic anemia patients with HLA-Haploidentical transplantation. Transplant Cell Ther 2023. https://doi.org/10.1016/j.jtct.2023.04.015.
DeZern AE, Zahurak M, Symons HJ, Cooke KR, Huff CA, Jain T et al. Alternative donor BMT with post-transplant cyclophosphamide as initial therapy for acquired severe aplastic anemia. Blood 2023. https://doi.org/10.1182/blood.2023020435.
Gong S, Chen C, Chen K, Yang R, Wang L, Yang K, et al. Alternative transplantation with post-transplantation cyclophosphamide in aplastic anemia: a retrospective report from the BMF-WG of Hunan province, China. Transpl Cell Ther. 2023;29:48.e1–48.e7.
George B, PN N, Devasia AJ, Kulkarni U, Korula A, Lakshmi KM, et al. Post-transplant cyclophosphamide as sole graft-versus-host disease prophylaxis is feasible in patients undergoing peripheral blood stem cell transplantation for severe aplastic anemia using matched sibling donors. Biol Blood Marrow Transpl. 2018;24:494–500.
Arcuri LJ, Nabhan SK, Loth G, Atta EH, Oliveira M, Nichele S, et al. A case series of post-transplantation cyclophosphamide in unrelated donor hematopoietic cell transplantation for aplastic anemia. Biol Blood Marrow Transpl. 2020;26:e222–6.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transpl. 1995;825–8.
Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–17.
MacMillan ML, Weisdorf DJ, Wagner JE, DeFor TE, Burns LJ, Ramsay NKC, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: Comparison of grading systems. Biol Blood Marrow Transpl. 2002;8:387–94.
Arcuri LJ, Nabhan SK, Cunha R, Nichele S, Ribeiro AAF, Fernandes JF, et al. Impact of CD34 Cell dose and conditioning regimen on outcomes after haploidentical donor hematopoietic stem cell transplantation with post-transplantation cyclophosphamide for relapsed/refractory severe Aplastic Anemia. Biol Blood Marrow Transpl. 2020;26:2311–7.
Prata PH, Eikema D-J, Afansyev B, Bosman P, Smiers F, Diez-Martin JL, et al. Haploidentical transplantation and posttransplant cyclophosphamide for treating aplastic anemia patients: a report from the EBMT Severe Aplastic Anemia Working Party. Bone Marrow Transpl. 2020;55:1050–8.
Hashem H, Rihani R, Shanap MA, Khattab E, Tbakhi A, Sultan I. Novel conditioning regimen for upfront haploidentical hematopoietic cell transplantation in children with severe aplastic anemia and donor-specific anti-HLA antibodies. Bone Marrow Transpl. 2022;57:304–5.
Kharya G, Jaiswal SR, Bhat S, Raj R, Yadav SP, Dua V, et al. Impact of conditioning regimen and graft-versus-host disease prophylaxis on the outcome of haploidentical peripheral blood stem cell transplantation for high-risk severe aplastic anemia in children and young adults: a report from the pediatric severe aplastic anemia consortium of India. Transplant Cell Ther. 2023;29:199.e1–199.e10.
Horan J, Wang T, Haagenson M, Spellman SR, Dehn J, Eapen M, et al. Evaluation of HLA matching in unrelated hematopoietic stem cell transplantation for nonmalignant disorders. Blood. 2012;120:2918–24.
Passweg JR, Pérez WS, Eapen M, Camitta BM, Gluckman E, Hinterberger W, et al. Bone marrow transplants from mismatched related and unrelated donors for severe aplastic anemia. Bone Marrow Transpl. 2006;37:641–9.
Lima ACM, Bonfim C, Getz J, do Amaral GB, Petterle RR, Loth G, et al. Untreated Donor-Specific HLA Antibodies are associated with graft failure and poor survival after haploidentical transplantation with post-transplantation cyclophosphamide in pediatric patients with nonmalignant disorders. Transplant Cell Ther. 2022;28:698.e1–698.e11.
Bolaños-Meade J, Cooke KR, Gamper CJ, Ali SA, Ambinder RF, Borrello IM, et al. Effect of increased dose of total body irradiation on graft failure associated with HLA-haploidentical transplantation in patients with severe haemoglobinopathies: a prospective clinical trial. Lancet Haematol. 2019;6:e183–93.
DeZern AE, Zahurak M, Jones RJ, Brodsky RA. Uniform conditioning regardless of donor in bone marrow transplantation for severe aplastic anemia. Haematologica. 2023;109:657–60.
Deeg HJ, Sociέ G, Schoch G, Henry-Amar M, Witherspoon RP, Devergie A, et al. Malignancies after marrow transplantation for aplastic anemia and fanconi anemia: a joint seattle and paris analysis of results in 700 patients. Blood. 1996;87:386–92.
Socie G, Henry-Amar M, Bacigalupo A, Hows J, Tichelli A, Ljungman P, et al. Malignant tumors occurring after treatment of aplastic anemia. N Engl J Med. 1993;329:1152–7.
Champlin RE, Perez WS, Passweg JR, Klein JP, Camitta BM, Gluckman E, et al. Bone marrow transplantation for severe aplastic anemia: a randomized controlled study of conditioning regimens. Blood. 2007;109:4582–5.
Bacigalupo A, Socié G, Schrezenmeier H, Tichelli A, Locasciulli A, Fuehrer M, et al. Bone marrow versus peripheral blood as the stem cell source for sibling transplants in acquired aplastic anemia: survival advantage for bone marrow in all age groups. Haematologica. 2012;97:1142–8.
Bacigalupo A, Socie’ G, Lanino E, Prete A, Locatelli F, Locasciulli A, et al. Fludarabine, cyclophosphamide, antithymocyte globulin, with or without low dose total body irradiation, for alternative donor transplants, in acquired severe aplastic anemia: a retrospective study from the EBMT-SAA working party. Haematologica. 2010;95:976–82.
Samarasinghe S, Clesham K, Iacobelli S, Sbianchi G, Knol C, Hamladji R, et al. Impact of T‐cell depletion strategies on outcomes following hematopoietic stem cell transplantation for idiopathic aplastic anemia: A study on behalf of the European blood and marrow transplant severe aplastic anemia working party. Am J Hematol. 2019;94:80–6.
Bacigalupo A, Brand R, Oneto R, Bruno B, Sodé G, Passweg J, et al. Treatment of acquired severe aplastic anemia: Bone marrow transplantation compared with immunosuppressive therapy-the European group for blood and marrow transplantation experience. Semin Hematol. 2000;37:69–80.
Shaffer BC, Gooptu M, DeFor TE, Maiers M, Bolaños-Meade J, Abboud R, et al. Post-transplant cyclophosphamide–based graft-versus-host disease prophylaxis attenuates disparity in outcomes between use of matched or mismatched unrelated donors. J Clin Oncol. 2024;42:3277–86.
Montoro J, Boumendil A, Finel H, Bramanti S, Castagna L, Blaise D et al. Post-transplant cyclophosphamide-based graft-versus-host disease prophylaxis in HLA-matched and haploidentical donor transplants for patients with Hodgkin lymphoma: a comparative study of the LWP EBMT. GVHD prophylaxis for patients with Hodgkin lymphoma. Transpl Cellular Therapy 2023. https://doi.org/10.1016/j.jtct.2023.11.021.
Lum SH, Albert MH, Gilbert P, Sirait T, Algeri M, Muratori R, et al. Outcomes of HLA-mismatched HSCT with TCRαβ/CD19 depletion or post-HSCT cyclophosphamide for inborn errors of immunity. Blood. 2024;144:565–80.
Morales E, Pulsipher MA. A better approach to mismatched HSCT than PTCY? Blood. 2024;144:474–6.
Acknowledgements
The authors are particularly thankful to all centers from the Severe Aplastic Anemia Working Party of the European Society for Blood and Marrow Transplantation, who kindly agreed to participate in this study.
Author information
Authors and Affiliations
Contributions
Conception and design: JM, AR, RPDL. Data analysis and interpretation: JM, DJE, AR, RPDL. Manuscript writing: JM. Final approval of manuscript: JM, DJE, BP, JT, KH, AK, AA, BAA, PR, MIR, ZG, AM, SA, MI, MR, GK, VP, MG, TS, SG, AB, MA, AH, JHD, JV, JS, JAPS, AC, MC, AT, CH, AK, AR, RPDL.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Patients prospectively provided signed informed consent for both data collection through the ProMISe system and any posteriori analysis. The study was conducted in accordance with the Declaration of Helsinki and was approved by the scientific committee of the SAAWP of the EBMT.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Montoro, J., Eikema, DJ., Piepenbroek, B. et al. Donor impact on allogeneic transplant outcomes with PTCy for severe aplastic anemia: a study of the SAAWP EBMT. Bone Marrow Transplant 60, 1152–1159 (2025). https://doi.org/10.1038/s41409-025-02633-y
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41409-025-02633-y