Abstract
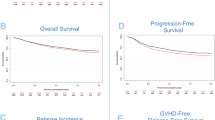
Allogeneic hematopoietic cell transplantation (alloHCT) from 8/10 HLA-matched unrelated donor is performed in a minority of patients. There is little data on its outcomes and consequently, guidelines on optimal transplantation procedures are lacking. The Transplant complications working party of the EBMT performed a registry study comparing approaches to graft-versus-host disease (GVHD) prophylaxis in recipients of alloHCT from 8/10 HLA-mismatched unrelated donors (8/10 MMUD). The analysis included 450 adult patients with hematological malignancies receiving a first alloHCT between 2015 and 2021, GVHD prophylaxis strategies included ATG in 318 and PTCy in 132 patients. AlloHCT from 8/10 MMUD resulted in 21.1% non-relapse mortality, 28.5% cumulative incidence of relapse, 55.7% overall survival (OS), 50.4% progression-free-survival and 39.8% GVHD-relapse-free survival (GRFS). PTCy decreased the risk of grade II-IV (HR 0.63, 95%CI 0.40–0.99) and III–IV acute GVHD (HR 0.31, 95%CI 0.13–0.74), improved OS (HR 0.63, 95%CI 0.41–0.95) and GRFS (HR 0.65, 95%CI 0.47–0.91). No other differences between the groups were documented. Transplantation from female donors to male recipients increased the incidence of extensive chronic GVHD (HR 2.39, 95%CI 1.10–5.19) and decreased PFS (HR 1.49, 95%CI 1.01–2.20). AlloHCT from 8/10 HLA-matched unrelated donor is feasible and the use of PTCy in GVHD prophylaxis improves outcomes.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Primary study data is available through partnering@ebmt.org.
References
Passweg JR, Baldomero H, Ciceri F, de la Cámara R, Glass B, Greco R, et al. Hematopoietic cell transplantation and cellular therapies in Europe 2022. CAR-T activity continues to grow; transplant activity has slowed: a report from the EBMT. Bone Marrow Transpl. 2024;59:803–12. https://doi.org/10.1038/s41409-024-02248-9.
Cusatis R, Litovich C, Feng Z, Allbee-Johnson M, Kapfhammer M, Mattila D, et al. Current trends and outcomes in cellular therapy activity in the United States, including prospective Patient Reported Outcomes data collection within the CIBMTR registery. Transplant Cell Ther. 2024;30:917.e1–917.e12. https://doi.org/10.1016/j.jtct.2024.06.021.
Ayuk F, Beelen DW, Bornhäuser M, Stelljes M, Zabelina T, Finke J, et al. Relative impact of HLA matching and Non-HLA donor characteristics on outcomes of allogeneic stem cell transplantation for acute myeloid leukemia and myelodysplastic syndrome. Biol Blood Marrow Transpl. 2018;24:2558–67. https://doi.org/10.1016/j.bbmt.2018.06.026.
Lorentino F, Labopin M, Bernardi M, Ciceri F, Socié G, Cornelissen JJ, et al. Acute leukemia working party of the european society for blood and marrow transplantation. Comparable outcomes of haploidentical, 10/10 and 9/10 unrelated donor transplantation in adverse karyotype AML in first complete remission. Am J Hematol. 2018;93:1236–44. https://doi.org/10.1002/ajh.25231.
Battipaglia G, Labopin M, Kröger N, Vitek A, Afanasyev B, Hilgendorf I, et al. Posttransplant cyclophosphamide vs antithymocyte globulin in HLA-mismatched unrelated donor transplantation. Blood. 2019;134:892–9. https://doi.org/10.1182/blood.2019000487.
Penack O, Abouqateb M, Peczynski C, Boreland W, Gülbas Z, Gedde-Dahl T, et al. PTCy versus ATG as graft-versus-host disease prophylaxis in mismatched unrelated stem cell transplantation. Blood Cancer J. 2024;14:45 https://doi.org/10.1038/s41408-024-01032-8.
Aljurf M, Weisdorf D, Alfraih F, Szer J, Müller C, Confer D, et al. “Worldwide Network for Blood & Marrow Transplantation (WBMT) special article, challenges facing emerging alternate donor registries”. Bone Marrow Transpl. 2019;54:1179–88. https://doi.org/10.1038/s41409-019-0476-6.
Ciurea SO, Cao K, Fernandez-Vina M, Kongtim P, Malki MA, Fuchs E, et al. The European Society for Blood and Marrow Transplantation (EBMT) consensus guidelines for the detection and treatment of donor-specific anti-hla antibodies (DSA) in haploidentical hematopoietic cell transplantation. Bone Marrow Transpl. 2018;53:521–34.
Mehta RS, Ramdial J, Marin D, Alousi A, Kanakry CG, Champlin RE, et al. Impact of donor age in haploidentical-post-transplantation cyclophosphamide versus matched unrelated donor post-transplantation cyclophosphamide hematopoietic stem cell transplantation in patients with acute myeloid leukemia. Transpl Cell Ther. 2023;29:377.e1–377.e7. https://doi.org/10.1016/j.jtct.2023.03.028.
Kasamon YL, Ambinder RF, Fuchs EJ, Zahurak M, Rosner GL, Bolaños-Meade J, et al. Prospective study of nonmyeloablative, HLA-mismatched unrelated BMT with high-dose posttransplantation cyclophosphamide. Blood Adv. 2017;1:288–92. https://doi.org/10.1182/bloodadvances.2016002766
Rappazzo KC, Zahurak M, Bettinotti M, Ali SA, Ambinder AJ, Bolaños-Meade J, et al. Nonmyeloablative, HLA-Mismatched Unrelated peripheral blood transplantation with high-dose post-transplantation cyclophosphamide. Transpl Cell Ther. 2021;27:909.e1–e6. https://doi.org/10.1016/j.jtct.2021.08.013.
Atsuta Y, Kato S, Morishima Y, Ohashi K, Fukuda T, Ozawa Y, et al. HLA Working Group of the Japan Society for Hematopoietic Cell Transplantation. Comparison of HLA allele mismatch and antigen mismatch in unrelated bone marrow transplantation in patients with leukemia. Biol Blood Marrow Transpl. 2019;25:436–42. https://doi.org/10.1016/j.bbmt.2018.10.002.
Shaw BE, Jimenez-Jimenez AM, Burns LJ, Logan BR, Khimani F, Shaffer BC, et al. National marrow donor program-sponsored multicenter, Phase II trial of HLA-mismatched unrelated donor bone marrow transplantation using post-transplant cyclophosphamide. J Clin Oncol. 2021;39:1971–82. https://doi.org/10.1200/JCO.20.03502.
Peters C, Schrauder A, Schrappe M, von Stackelberg A, Stary J, Yaniv I, et al. BFM Study Group, the IBFM-Study Group and the Paediatric Disease Working Party of the EBMT. Allogeneic haematopoietic stem cell transplantation in children with acute lymphoblastic leukaemia: the BFM/IBFM/EBMT concepts. Bone Marrow Transpl. 2005;35 Suppl 1:S9–11. https://doi.org/10.1038/sj.bmt.1704835.
Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the disease risk index for allogeneic stem cell transplantation. Blood. 2014;123:3664–71. https://doi.org/10.1182/blood-2014-01-552984.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9.
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. https://doi.org/10.1080/01621459.1999.10474144
Passweg JR, Baldomero H, Ciceri F, Corbacioglu S, de la Cámara R, Dolstra H, et al. Hematopoietic cell transplantation and cellular therapies in Europe 2021. The second year of the SARS-CoV-2 pandemic. A Report from the EBMT Activity Survey. Bone Marrow Transpl. 2023;58:647–58. https://doi.org/10.1038/s41409-023-01943-3.
Sureda A, Corbacioglu S, Greco R, Kröger N, Carreras E, editors. The EBMT Handbook: hematopoietic stem cell transplantation and cellular therapies. 8th ed. Cham, Switzerland: Springer Nature Switzerland; 2024.
Rubio MT, Savani BN, Labopin M, Polge E, Niederwieser D, Ganser A, et al. The impact of HLA-matching on reduced intensity conditioning regimen unrelated donor allogeneic stem cell transplantation for acute myeloid leukemia in patients above 50 years-a report from the EBMT acute leukemia working party. J Hematol Oncol. 2016;9:65 https://doi.org/10.1186/s13045-016-0295-9.
Devillier R, Dalle JH, Kulasekararaj A, D’aveni M, Clément L, Chybicka A, et al. Unrelated alternative donor transplantation for severe acquired aplastic anemia: a study from the French Society of bone marrow transplantation and cell therapies and the EBMT severe aplastic anemia working party. Haematologica. 2016;101:884–90. https://doi.org/10.3324/haematol.2015.138727.
Meurer T, Crivello P, Metzing M, Kester M, Megger DA, Chen W, et al. Permissive HLA-DPB1 mismatches in HCT depend on immunopeptidome divergence and editing by HLA-DM. Blood. 2021;137:923–8. https://doi.org/10.1182/blood.2020008464.
Arrieta-Bolaños E, van der Burg LLJ, Gedde-Dahl T, Robin M, Salmenniemi U, Kroeger N, et al. Directionality of HLA-DP permissive mismatches improves risk prediction in HCT for acute leukemia and MDS. Blood. 2024;144:1747–51. https://doi.org/10.1182/blood.2024024351.
Piemontese S, Ciceri F, Labopin M, Bacigalupo A, Huang H, Santarone S, et al. Acute Leukemia Working Party (ALWP) of the European Group for Blood and Marrow Transplantation (EBMT). A survey on unmanipulated haploidentical hematopoietic stem cell transplantation in adults with acute leukemia. Leukemia 2015;29:1069–75. https://doi.org/10.1038/leu.2014.336.
Penack O, Abouqateb M, Peczynski C, Boreland W, Kröger N, Stelljes M, et al. ATG or post-transplant cyclophosphamide to prevent GVHD in matched unrelated stem cell transplantation? Leukemia. 2024;38:1156–63. https://doi.org/10.1038/s41375-024-02225-7.
Bolaños-Meade J, Hamadani M, Wu J, Al Malki MM, Martens MJ, Runaas L, et al. BMT CTN 1703 investigators. post-transplantation cyclophosphamide-based graft-versus-host disease prophylaxis. N. Engl J Med. 2023;388:2338–48. https://doi.org/10.1056/NEJMoa2215943.
Kröger N, Solano C, Wolschke C, Bandini G, Patriarca F, Pini M, et al. Antilymphocyte globulin for prevention of chronic graft-versus-host disease. N. Engl J Med. 2016;374:43–53. https://doi.org/10.1056/NEJMoa1506002.
Fletcher RE, Nunes NS, Patterson MT, Vinod N, Khan SM, Mendu SK, et al. Posttransplantation cyclophosphamide expands functional myeloid-derived suppressor cells and indirectly influences Tregs. Blood Adv. 2023;7:1117–29. https://doi.org/10.1182/bloodadvances.2022007026.
Shelikhova L, Glushkova S, Nikolaev R, Dunaikina M, Zhekhovtsova Z, Blagov S, et al. Serotherapy-free regimen improves non-relapse mortality and immune recovery among the recipients of αβ Tcell-depleted haploidentical grafts: retrospective study in childhood leukemia. Transpl Cell Ther. 2021;27:330.e1–e9. https://doi.org/10.1016/j.jtct.2021.01.010.
Kanda J, Long GD, Gasparetto C, Horwitz ME, Sullivan KM, Chute JP, et al. Reduced-intensity allogeneic transplantation using alemtuzumab from HLA-matched related, unrelated, or haploidentical related donors for patients with hematologic malignancies. Biol Blood Marrow Transpl. 2014;20:257–63. https://doi.org/10.1016/j.bbmt.2013.11.010.
Spyridonidis A, Labopin M, Brissot E, Moiseev I, Cornelissen J, Choi G, et al. Should anti-thymocyte globulin be added in post-transplant cyclophosphamide based matched unrelated donor peripheral blood stem cell transplantation for acute myeloid leukemia? A study on behalf of the Acute Leukemia Working Party of the EBMT. Bone Marrow Transpl. 2022;57:1774–80. https://doi.org/10.1038/s41409-022-01816-1.
Nagler A, Labopin M, Mielke S, Passweg J, Blaise D, Gedde-Dahl T, et al. Matched related versus unrelated versus haploidentical donors for allogeneic transplantation in AML patients achieving first complete remission after two induction courses: a study from the ALWP/EBMT. Bone Marrow Transpl. 2023;58:791–800. https://doi.org/10.1038/s41409-023-01980-y.
Ruggeri A, Labopin M, Bacigalupo A, Afanasyev B, Cornelissen JJ, Elmaagacli A, et al. Post-transplant cyclophosphamide for graft-versus-host disease prophylaxis in HLA matched sibling or matched unrelated donor transplant for patients with acute leukemia, on behalf of ALWP-EBMT. J Hematol Oncol. 2018;11:40 https://doi.org/10.1186/s13045-018-0586-4.
Mehta RS, Rezvani K. Immune reconstitution post allogeneic transplant and the impact of immune recovery on the risk of infection. Virulence. 2016;7:901–16. https://doi.org/10.1080/21505594.2016.1208866.
Prabahran A, Koldej R, Chee L, Ritchie D. Clinical features, pathophysiology, and therapy of poor graft function post-allogeneic stem cell transplantation. Blood Adv. 2022;6:1947–59. https://doi.org/10.1182/bloodadvances.2021004537.
Sanz J, Galimard JE, Labopin M, Afanasyev B, Sergeevich MI, Angelucci E, et al. Post-transplant cyclophosphamide containing regimens after matched sibling, matched unrelated and haploidentical donor transplants in patients with acute lymphoblastic leukemia in first complete remission, a comparative study of the ALWP of the EBMT. J Hematol Oncol. 2021;14:84 https://doi.org/10.1186/s13045-021-01094-2.
Fernandez-Viña MA, Wang T, Lee SJ, Haagenson M, Aljurf M, Askar M, et al. Identification of a permissible HLA mismatch in hematopoietic stem cell transplantation. Blood. 2014;123:1270–8. https://doi.org/10.1182/blood-2013-10-532671.
Morishima Y, Kashiwase K, Matsuo K, Azuma F, Morishima S, Onizuka M, et al. Japan marrow donor program biological significance of HLA locus matching in unrelated donor bone marrow transplantation. Blood. 2015;125:1189–97.
Arrieta-Bolaños E, Bonneville EF, Crivello P, Robin M, Gedde-Dahl T, Salmenniemi U et al. Cellular therapy and immunobiology working party of the EBMT. Human leukocyte antigen mismatching and survival in contemporary hematopoietic cell transplantation for hematologic malignancies. J Clin Oncol. 2024:JCO2400582. https://doi.org/10.1200/JCO.24.00582.
Dehn J, Spellman S, Hurley CK, Shaw BE, Barker JN, Burns LJ, et al. Selection of unrelated donors and cord blood units for hematopoietic cell transplantation: guidelines from the NMDP/CIBMTR. Blood. 2019;134:924–34. https://doi.org/10.1182/blood.2019001212.
Author information
Authors and Affiliations
Contributions
I.M., C.P., and O.P. designed the study; M.A. performed the statistical analyses; I.M. wrote the manuscript; Z.P., O.P., W.B., C.P., H.S., C.G., and A.M. revised the manuscript; A.B., N.K., R.C., R.Z., T.S., M.P., R.P.L., W.B., J.V., D.B., M.E., and F.F. were the principal investigators at the centers recruiting the largest numbers of patients for the study; and all authors reviewed the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
I.M. reports having received honoraria from Novartis, Sanofi, J&J, Takeda; H.S. reports having received personal fees from Incyte, Janssen, Novartis, Sanofi and from the Belgian Hematological Society (BHS), as well as research grants from Novartis and the BHS, all paid to her institution and not directly related to this work. She has also received non-financial support (travel grants) from Gilead, Pfizer, the EBMT (European Society for Blood and Marrow transplantation) and the CIBMTR (Center for International Bone Marrow Transplantation Research). C.G. reports having received travel support from the EBMT and research funding from the American Association of Cancer Research (AACR).
Ethics declarations
All patients in all centers gave their written informed consent to use their personal and medical data for research purposes. Each center is responsible for obtaining such consent before uploading data to the EMBT database. The study was developed and approved by the Transplant Complications Working Party of the EBMT. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moiseev, I., Abouqateb, M., Peczynski, C. et al. Post-transplantation cyclophosphamide and antithymocyte globulin in 8/10 HLA-mismatched unrelated donor transplantation: the analysis on behalf of the transplant complications working party of the EBMT. Bone Marrow Transplant (2025). https://doi.org/10.1038/s41409-025-02678-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41409-025-02678-z