Abstract
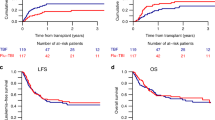
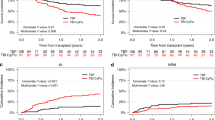
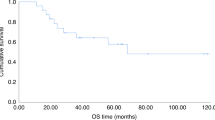
The optimal reduced-intensity conditioning (RIC) regimen for haploidentical hematopoietic stem cells transplantation (haplo-HSCT) using post-transplant cyclophosphamide as graft-versus-host disease (GVHD) prophylaxis has yet to be determined. Potential RIC regimen for haplo-HSCT in myeloid malignancies include Clofarabine-Baltimore (CloB) and TBF (thiotepa-busulfan-fludarabine). This multicenter retrospective study compared 297 adult patients receiving CloB (n = 59) or TBF (n = 238). The main diagnoses were acute myeloid leukemia (63%), 36% having adverse risk features. Median follow-up was 22.7 months. No significant differences were observed in overall (OS), progression-free (PFS), or GVHD-free relapse-free survival. However, 2-year non-relapse mortality (NRM) was higher after TBF (34% vs 21%, HR: 0.38; 95%CI: 0.20–0.75, p = 0.005), although the relapse incidence was lower (13% vs 23%, HR: 1.94; 95%CI: 0.98–3.87, p = 0.059). A 1:1 propensity score matching allowed the comparison of 53 CloB with 53 TBF. CloB was associated with improved 2-year OS (63% vs 44%, p = 0.02) due to a higher 2-year NRM in the TBF group (48% vs 19%, p = 0.002). By multivariate analysis, CloB remained associated with better OS (HR 0.52, 95% I 0.28–0.99, p = 0.045) and TBF with higher NRM (HR 3.43, 95%CI 1.59–7.41, p = 0.002). These results suggest that CloB is superior to TBF as a RIC regimen prior to haplo-HSCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Data from this study can be made available upon reasonable request from the corresponding author.
References
Passweg JR, Baldomero H, Ciceri F, de la Cámara R, Glass B, Greco R, et al. Hematopoietic cell transplantation and cellular therapies in Europe 2022. CAR-T activity continues to grow; transplant activity has slowed: a report from the EBMT. Bone Marrow Transpl. 2024;59:803–12.
Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transpl. 2008;14:641–50.
Radojcic V, Luznik L. Mechanism of action of posttransplantation cyclophosphamide: more than meets the eye. J Clin Investig. 2019;129:2189–91.
Arcuri LJ, Aguiar MTM, Ribeiro AAF, Pacheco AGF. Haploidentical Transplantation with Post-Transplant Cyclophosphamide versus Unrelated Donor Hematopoietic Stem Cell Transplantation: A Systematic Review and Meta-Analysis. Biol Blood Marrow Transpl. 2019;25:2422–30.
Rieger MJ, Stolz SM, Müller AM, Schwotzer R, Nair G, Schneidawind D, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched related and unrelated donor transplant in acute myeloid leukemia and myelodysplastic neoplasm. Bone Marrow Transpl. 2023;58:1121–9.
Srinivasan A, Raffa E, Wall DA, Schechter T, Ali M, Chopra Y, et al. Outcome of Haploidentical Peripheral Blood Allografts Using Post-Transplantation Cyclophosphamide Compared to Matched Sibling and Unrelated Donor Bone Marrow Allografts in Pediatric Patients with Hematologic Malignancies: A Single-Center Analysis. Transplant Cell Ther. 2022;28:158.e1–158.e9.
Nakaya Y, Koh H, Konuma T, Shimomura Y, Ishiyama K, Itonaga H, et al. HLA-Haploidentical Peripheral Blood Stem Cell Transplantation with Post-Transplantation Cyclophosphamide versus HLA-Matched Unrelated Donor Transplantation for Myelodysplastic Syndrome. Transplant Cell Ther. 2024;30:316.e1–316.e12.
Arcuri LJ, Ribeiro AAF, Hamerschlak N, Kerbauy MN. Posttransplant cyclophosphamide beyond haploidentical transplantation. Ann Hematol. 2024;103:1483–91.
Harbi S, Boher jeanM, Forcade E, Chevallier P, Régis PDL, Malard F, et al. Impact of Donor Search Strategy for Allogeneic Hematopoietic Stem Cell Transplantation from Haploidentical or Matched Unrelated Donor for Patients Older Than 55 Years with Hematological Malignancies: Randomized Prospective Phase III Study. Blood. 2020;136:35–36.
Munchel A, Kesserwan C, Symons HJ, Luznik L, Kasamon YL, Jones RJ, et al. Nonmyeloablative, HLA-haploidentical bone marrow transplantation with high dose, post-transplantation cyclophosphamide. Pediatr Rep. 2011;3:e15.
Sugita J, Yanada M. Current status of conditioning regimens in haploidentical hematopoietic cell transplantation. Hematology. 2024;29:2332866.
Chevallier P, Peterlin P, Garnier A, Le Bourgeois A, Mahé B, Dubruille V, et al. Clofarabine-based reduced intensity conditioning regimen with peripheral blood stem cell graft and post-transplant cyclophosphamide in adults with myeloid malignancies. Oncotarget. 2018;9:33528–35.
Duléry R, Bastos J, Paviglianiti A, Malard F, Brissot E, Battipaglia G, et al. Thiotepa, Busulfan, and Fludarabine Conditioning Regimen in T Cell-Replete HLA-Haploidentical Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transpl. 2019;25:1407–15.
Spyridonidis A, Labopin M, Savani BN, Niittyvuopio R, Blaise D, Craddock C, et al. Redefining and measuring transplant conditioning intensity in current era: a study in acute myeloid leukemia patients. Bone Marrow Transpl. 2020;55:1114–25.
Jullien M, Le Bourgeois A, Peterlin P, Garnier A, Guillaume T, Béné MC, et al. Addition of ATG to non-myeloablative peripheral blood haploidentical transplant with PTCY decreases acute GVHD rates and improves GVHD-relapse free survival. Bone Marrow Transpl. 2023;58:723–6.
El-Cheikh J, Devillier R, Dulery R, Massoud R, Al Chami F, Ghaoui N, et al. Impact of Adding Antithymocyte Globulin to Posttransplantation Cyclophosphamide in Haploidentical Stem-Cell Transplantation. Clin Lymphoma Myeloma Leuk. 2020;20:617–23.
Devillier R, Galimard J-E, Labopin M, Blaise D, Raiola AM, Pavlu J, et al. Reduced intensity versus non-myeloablative conditioning regimen for haploidentical transplantation and post-transplantation cyclophosphamide in complete remission acute myeloid leukemia: a study from the ALWP of the EBMT. Bone Marrow Transpl. 2022;57:1421–7.
Ruggeri A, Labopin M, Ciceri F, Mohty M, Nagler A. Definition of GvHD-free, relapse-free survival for registry-based studies: an ALWP–EBMT analysis on patients with AML in remission. Bone Marrow Transplant. 2016;51:610–1.
Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, Multicenter Standardization of Acute Graft-versus-Host Disease Clinical Data Collection: A Report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transpl. 2016;22:4–10.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transpl. 2015;21:389–401.e1.
Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal Am Stat Assoc. 1999;94:496–509.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9.
Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47.
Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–65.
Pagliardini T, Castagna L, Harbi S, Porta MD, Rey J, Fürst S, et al. Thiotepa, Fludarabine, and Busulfan Conditioning Regimen before T Cell-Replete Haploidentical Transplantation with Post-Transplant Cyclophosphamide for Acute Myeloid Leukemia: A Bicentric Experience of 100 Patients. Biol Blood Marrow Transpl. 2019;25:1803–9.
Saraceni F, Labopin M, Raiola AM, Blaise D, Reményi P, Sorà F, et al. Thiotepa-busulfan-fludarabine Compared to Treosulfan-based Conditioning for Haploidentical Transplant With Posttransplant Cyclophosphamide in Patients With Acute Myeloid Leukemia in Remission: A Study From the Acute Leukemia Working Party of the EBMT. Hemasphere. 2023;7:e952.
Beelen DW, Trenschel R, Stelljes M, Groth C, Masszi T, Reményi P, et al. Treosulfan or busulfan plus fludarabine as conditioning treatment before allogeneic haemopoietic stem cell transplantation for older patients with acute myeloid leukaemia or myelodysplastic syndrome (MC-FludT.14/L): a randomised, non-inferiority, phase 3 trial. Lancet Haematol. 2020;7:e28–39.
Cassanello G, Serpenti F, Bagnoli F, Saporiti G, Goldaniga M, Cavallaro F, et al. Treosulfan, thiotepa and fludarabine conditioning regimen prior to first allogeneic stem cell transplantation in acute myeloid leukemia and high-risk myelodysplastic syndromes: a single center experience. Bone Marrow Transpl. 2023;58:1059–61.
Duléry R, Malard F, Brissot E, Banet A, Sestili S, Belhocine R, et al. Reduced post-transplant cyclophosphamide dose with antithymocyte globulin in peripheral blood stem cell haploidentical transplantation. Bone Marrow Transpl. 2023;58:1215–22.
Raiola AM, Di Grazia C, Dominietto A, Bregante S, Giammarco S, Varaldo R, et al. Haploidentical bone marrow transplantation for AML in remission after TBF conditioning: a long-term follow-up. Blood Adv. 2024;8:1964–7.
Makanga DR, Guillaume T, Willem C, Legrand N, Gagne K, Cesbron A, et al. Posttransplant Cyclophosphamide and Antithymocyte Globulin versus Posttransplant Cyclophosphamide as Graft-versus-Host Disease Prophylaxis for Peripheral Blood Stem Cell Haploidentical Transplants: Comparison of T Cell and NK Effector Reconstitution. J Immunol. 2020;205:1441–8.
Passweg JR, Tichelli A, Meyer-Monard S, Heim D, Stern M, Kühne T, et al. Purified donor NK-lymphocyte infusion to consolidate engraftment after haploidentical stem cell transplantation. Leukemia. 2004;18:1835–8.
Prin Felix L Treosulfan Vs Busulfan As Part of Clofarabine Based Reduced Intensity Conditioning Regimen before Allotransplant for Myeloid Malignancies. ASH, 2024 https://ash.confex.com/ash/2024/webprogram/Paper203266.html (accessed 6 Dec2024).
Sharma A, Kang G, Sunkara A, Inaba H, Jeha S, Cross SJ, et al. Haploidentical Donor Transplantation Using a Novel Clofarabine-containing Conditioning Regimen for Very High-risk Hematologic Malignant Neoplasms. J Pediatr Hematol Oncol. 2018;40:e479–85.
Santoro N, Mooyaart JE, Devillier R, Koc Y, Vydra J, Castagna L, et al. Donor lymphocyte infusions after haploidentical stem cell transplantation with PTCY: A study on behalf of the EBMT cellular therapy & immunobiology working party. Bone Marrow Transpl. 2023;58:54–60.
Guillaume T, Thépot S, Peterlin P, Ceballos P, Bourgeois AL, Garnier A, et al. Prophylactic or Preemptive Low-Dose Azacitidine and Donor Lymphocyte Infusion to Prevent Disease Relapse following Allogeneic Transplantation in Patients with High-Risk Acute Myelogenous Leukemia or Myelodysplastic Syndrome. Transplant Cell Ther. 2021;27:839.e1–839.e6.
Garcia JS, Kim HT, Murdock HM, Ansuinelli M, Brock J, Cutler CS, et al. Prophylactic maintenance with venetoclax/azacitidine after reduced-intensity conditioning allogeneic transplant for high-risk MDS and AML. Blood Adv. 2024;8:978–90.
Lacan C, Lambert J, Forcade E, Robin M, Chevallier P, Loron S, et al. Bone marrow graft versus peripheral blood graft in haploidentical hematopoietic stem cells transplantation: a retrospective analysis in1344 patients of SFGM-TC registry. J Hematol Oncol. 2024;17:2.
Spyridonidis A, Labopin M, Brissot E, Moiseev I, Cornelissen J, Choi G, et al. Should anti-thymocyte globulin be added in post-transplant cyclophosphamide based matched unrelated donor peripheral blood stem cell transplantation for acute myeloid leukemia? A study on behalf of the Acute Leukemia Working Party of the EBMT. Bone Marrow Transpl. 2022;57:1774–80.
Ciceri F, Bacigalupo A, Lankester A, Bertaina A Haploidentical HSCT. In: Carreras E, Dufour C, Mohty M, Kröger N (eds). The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies. Springer: Cham (CH), 2019 http://www.ncbi.nlm.nih.gov/books/NBK553970/ (accessed 4 Mar2024).
Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123:3664–71.
Acknowledgements
We thank Nicole Raus and Viviane Fossat, data manager from the SFGM-TC. Medical writing for this manuscript was assisted by MPIYP (MC Béné), Paris, France.
Author information
Authors and Affiliations
Consortia
Contributions
MJ and PC designed the study, interpreted the data and wrote the manuscript. MJ performed all the statistical analysis. All coauthors contributed data to the EBMT registry, reviewed the article, and approved the final version. PC and MJ conceived and designed the study, interpreted the results and drafted the manuscript. MJ performed the statistical analyses and generated the figures. EB, ED, ML, AB, HLW, AH, AST, AC, PT, FB, RD, JOB, MS, PCe, MA, LS, TM, MTR, EF, JC, SC, CEB, AV, AG, PP, ALB, TG, contributed to patient care and data collection. All authors critically revised the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was approved by the Institutional Review Board of the SFGM-TC. All methods were performed in accordance with the relevant guidelines and regulations.
Patient consent statement
Informed consent was obtained from all patients included in this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jullien, M., Brissot, E., Daguindau, E. et al. Thiotepa-busulfan-fludarabine compared to clofarabine-based conditioning for haploidentical transplant with posttransplant cyclophosphamide in patients with myeloid malignancies: a retrospective study from the SFGM-TC. Bone Marrow Transplant 60, 1592–1600 (2025). https://doi.org/10.1038/s41409-025-02709-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41409-025-02709-9