Abstract
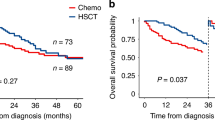
Despite the increasing number of cancer survivors, the impact of prior solid tumors and their treatment modality on outcomes after allogeneic hematopoietic stem cell transplantation (allo-HSCT) remains unclear. This multi-center retrospective study compared transplant outcomes in allo-HSCT recipients with and without a history of solid tumors. Of 5,850 adult patients who underwent first allo-HSCT, 303 (5.2%) had a prior solid tumor. After propensity score matching, overall survival (OS) and cumulative incidences of relapse, non-relapse mortality (NRM), and acute and chronic graft-versus-host diseases were almost comparable between the two groups. Progression or recurrence of solid tumors after allo-HSCT occurred in only eight cases (2.8%). Importantly, patients who received both chemotherapy and radiation therapy (Chemo + RT) for prior solid tumors had significantly worse OS (35.3% vs. 54.1% [P < 0.001] vs. 56.8% [P < 0.001] at 2 years) and higher NRM (36.2% vs. 18.7% [P = 0.002] vs. 19.3% [P = 0.001] at 2 years) compared to patients who received other treatment modalities for prior solid tumors or patients without prior solid tumors. These findings highlight the feasibility of allo-HSCT in selected patients with prior solid tumors, while demonstrating the negative impact of Chemo + RT on outcomes after allo-HSCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
References
Majhail NS, Farnia SH, Carpenter PA, Champlin RE, Crawford S, Marks DI, et al. Indications for Autologous and Allogeneic Hematopoietic Cell Transplantation: Guidelines from the American Society for Blood and Marrow Transplantation. Biology Blood Marrow Transplant. 2015;21:1863–9.
Kanate AS, Majhail NS, Savani BN, Bredeson C, Champlin RE, Crawford S, et al. Indications for Hematopoietic Cell Transplantation and Immune Effector Cell Therapy: Guidelines from the American Society for Transplantation and Cellular Therapy. Biology Blood Marrow Transplant. 2020;26:1247–56.
Tabbara IA, Zimmerman K, Morgan C, Nahleh Z. Allogeneic Hematopoietic Stem Cell Transplantation: Complications and Results. Arch Intern Med. 2002;162:1558–66.
McDonald GB, Sandmaier BM, Mielcarek M, Sorror M, Pergam SA, Cheng G-S, et al. Survival, nonrelapse mortality, and relapse-related mortality after allogeneic hematopoietic cell transplantation: Comparing 2003–2007 versus 2013–2017 cohorts. Ann Intern Med. 2020;172:229–39.
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–63.
Godley LA, Larson RA. Therapy-Related Myeloid Leukemia. Seminars Oncol. 2008;35:418–29.
McNerney ME, Godley LA, le Beau MM. Therapy-related myeloid neoplasms: When genetics and environment collide. Nature Rev Cancer. 2017;17:513–27.
Morton LM, Dores GM, Schonfeld SJ, Linet MS, Sigel BS, Lam CJK, et al. Association of Chemotherapy for Solid Tumors with Development of Therapy-Related Myelodysplastic Syndrome or Acute Myeloid Leukemia in the Modern Era. JAMA Oncol. 2019;5:318–25.
Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–75.
Singhal D, Kutyna MM, Hahn CN, Shah MV, Hiwase DK. Therapy-Related Myeloid Neoplasms: Complex Interactions among Cytotoxic Therapies, Genetic Factors, and Aberrant Microenvironment. Blood Cancer Discov. 2024;5:400–16.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9.
Kataoka K, Nannya Y, Ueda K, Kumano K, Takahashi T, Kurokawa M. Differential prognostic impact of pretransplant comorbidity on transplant outcomes by disease status and time from transplant: A single Japanese transplant centre study. Bone Marrow Transplant. 2010;45:513–20.
Shouval R, Fein JA. The sum of the parts: what we can and cannot learn from comorbidity scores in allogeneic transplantation. Hematology Am Soc Hematol Educ Program. 2023;2023:715–22.
Katanoda K, Hori M, Saito E, Shibata A, Ito Y, Minami T, et al. Updated trends in cancer in japan: Incidence in 1985–2015 and mortality in 1958–2018—a sign of decrease in cancer incidence. J Epidemiol. 2021;31:426–50.
Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annual Rev Immunol. 2007;25:139–70.
Jenq RR, van den Brink MRM. Allogeneic haematopoietic stem cell transplantation: Individualized stem cell and immune therapy of cancer. Nature Rev Cancer. 2010;10:213–21.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transpl. 1995;15:825–8.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group Report. Biology Blood Marrow Transplant. 2015;21:389–401.e1.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the Intensity of Conditioning Regimens: Working Definitions. Biology Blood Marrow Transplant. 2009;15:1628–33.
Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-Intensity Conditioning Regimen Workshop: Defining the Dose Spectrum. Report of a Workshop Convened by the Center for International Blood and Marrow Transplant Research. Biology Blood Marrow Transplant. 2009;15:367–9.
Singh ZN, Huo D, Anastasi J, Smith SM, Karrison T, Beau MML, et al. Therapy-related myelodysplastic syndrome: morphologic subclassification may not be clinically relevant. Am J Clin Pathol. 2007;127:197–205.
Morton LM, Dores GM, Tucker MA, Kim CJ, Onel K, Gilbert ES, et al. Evolving risk of therapy-related acute myeloid leukemia following cancer chemotherapy among adults in the United States, 1975-2008. Blood. 2013;121:2996–3004.
D’Agostino RB. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–81.
Chen JW, Maldonado DR, Kowalski BL, Miecznikowski KB, Kyin C, Gornbein JA, et al. Best Practice Guidelines for Propensity Score Methods in Medical Research: Consideration on Theory, Implementation, and Reporting. A Review. Arthroscopy. 2022;38:632–42.
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424.
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transpl. 2013;48:452–8.
Portuguese AJ, Albittar A, Gooley TH, Deeg HJ. Transplantation for myeloid neoplasms with antecedent solid tumor. Cancer. 2023;129:142–50.
Mauch P, Constine L, Greenberger J, Knospe W, Sullivan J, Liesveld JL, et al. Hematopoietic stem cell compartment: Acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys. 1995;31:1319–39.
Shao L, Luo Y, Zhou D. Hematopoietic stem cell injury induced by ionizing radiation. Antioxidants Redox Signal. 2014;20:1447–62.
Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nature Med. 2014;20:833–46.
Srinivas US, Tan BWQ, Vellayappan BA, Jeyasekharan AD. ROS and the DNA damage response in cancer. Redox Biol. 2019;25:101084.
Groelly FJ, Fawkes M, Dagg RA, Blackford AN, Tarsounas M. Targeting DNA damage response pathways in cancer. Nature Rev Cancer. 2023;23:78–94.
Gradishar WJ, Moran MS, Abraham J, Abramson V, Aft R, Agnese D, et al. Breast Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2024;22:331–57.
Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32:1267–84.
Mellman I, Chen DS, Powles T, Turley SJ. The cancer-immunity cycle: Indication, genotype, and immunotype. Immunity. 2023;56:2188–205.
Kayser S, Dö HK, Rgen Krauter J, Köhne C-H, Horst HA, Held G, et al. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood. 2011;117:2137–45.
Madanat YF, Gerds AT. Can allogeneic hematopoietic cell transplant cure therapy-related acute leukemia?. Best Pr Res Clin Haematol. 2019;32:104–13.
Acknowledgements
The authors thank all patients who participated in the study and all members of Kanto Study of Group for Cell Therapy (KSGCT). The authors also thank Toyohiro Kawano and the members of the KSGCT data center for data management.
Funding
The authors received no specific financial support for this work.
Author information
Authors and Affiliations
Contributions
TF, MS and KK designed the study, performed the analyses, and wrote the manuscript. HS, AI, KK, KS, ST, YO, SK, SM, ES, AJ, FO, YN, TK, TT, MS, SI, MO, SY, KH, MH, NA, SF, ST, NK, TK and KT contributed to data collection and revised the manuscript. KK and YK supervised the study. All authors discussed the results and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
KK received honoraria from Ono Pharmaceutical, Eisai, Astellas Pharma, Novartis, Chugai Pharmaceutical, AstraZeneca, Sumitomo Pharma, Kyowa Kirin, Janssen Pharmaceutical, Takeda Pharmaceutical, Otsuka Pharmaceutical, SymBio Pharmaceuticals, Bristol Myers Squibb, Pfizer, Nippon Shinyaku, Daiichi Sankyo, Alexion Pharmaceuticals, AbbVie, Meiji Seika Pharma, Sanofi, Sysmex, Mundipharma, Incyte Corporation, and Kyorin Pharmaceutical. KK received research support from Otsuka Pharmaceutical, Chordia Therapeutics, Chugai Pharmaceutical, Takeda Pharmaceutical, and Meiji Seika Pharma. KK received scholarship from Asahi Kasei Pharma, Eisai, Otsuka Pharmaceutical, Ono Pharmaceutical, Kyowa Kirin, Shionogi, Takeda Pharmaceutical, Sumitomo Dainippon Pharma, Chugai Pharmaceutical, Teijin Pharma, Japan Blood Products Organization, Mochida Pharmaceutical, JCR Pharmaceuticals, and Nippon Shinyaku. KK owns stock in Asahi Genomics. KK has a patent for Genetic alterations as a biomarker in T-cell lymphomas and a patent for PD-L1 abnormalities as a predictive biomarker for immune checkpoint blockade therapy. SF reports honoraria from Bristol-Myers-Squibb, Nippon Shinyaku, Otsuka Pharmaceutical Co., Ltd., Pfizer Japan Inc., Novartis Pharma KK, Janssen, Kyowa Kirin Co., Ltd., AstraZeneca, CSL Behring K.K, Meiji Seika Pharma, AbbVie Inc, Takeda Pharmaceutical Co., Ltd., Asahi Kasei Pharma Co., Ltd., Daiichi Sankyo Co., Ltd., Kissei, PharmaEssentia Japan, Genmab, Argenx Japan, Alexion Pharma, Inc., and Chugai Pharmaceutical Co., Ltd. and research funding from Shionogi Co., Ltd., Chugai Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Asahi-Kasei Pharma, and Daiichi Sankyo Co., Ltd., outside of the submitted work.
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of Keio University School of Medicine (Tokyo, Japan) (approval number 20231134). All patients provided informed consent for the use of their clinical data for research purposes. No identifiable images or other personal details of participants are presented in this manuscript.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fujii, T., Sakurai, M., Shimizu, H. et al. Impact of prior solid tumors and their treatment modality on outcomes after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant (2025). https://doi.org/10.1038/s41409-025-02718-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41409-025-02718-8