Abstract
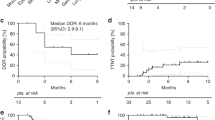
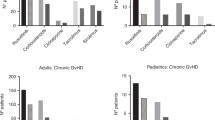
Belumosudil is approved after failure of ≥2 lines of therapy in chronic graft-versus-host disease cGVHD. However, real-world data is limited. We conducted a retrospective analysis of 67 patients with steroid-refractory or dependent (SR/SD) cGVHD. At baseline, most patients had advanced multi-organ cGVHD. The 6- and 12-month overall response rate (ORR) was 61%. However, a subset of patients achieved deeper responses with ongoing therapy at 12 months. The 6-month failure-free survival (FFS) was 75% (95%CI: 65–86) whereas the 12-month FFS was 66% (95%CI: 55–78). A low incidence of drug-related grade ≥3 toxicities was observed. A cohort of patients with immune function analysis showed gradual improvement in immune subsets at 1-year post-treatment. The combined bel-rux cohort (n = 14) showed a 6- and 12-month ORR of 64% and 57%, respectively. Overall, belumosudil was associated with high treatment response and survival outcomes. Notably, deeper responses were observed with ongoing therapy, and it was overall well tolerated. In a cohort of patients, immune cell populations had preserved to improved values throughout treatment. Patients who received bel-rux demonstrated efficacy and safety as well. Overall, our real-world study indicates similar findings to the clinical trial and supports the use of belumosudil in cGVHD.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on a reasonable request.
References
Zeiser R, Blazar BR. Pathophysiology of chronic graft-versus-host disease and therapeutic targets. N Engl J Med. 2017;377:2565–79.
Wong FL, Francisco L, Togawa K, Bosworth A, Gonzales M, Hanby C, et al. Long-term recovery after hematopoietic cell transplantation: predictors of quality-of-life concerns. Blood. 2010;115:2508–19.
Pidala J, Kurland B, Chai XY, Majhail N, Weisdorf D, Pavletic S, et al. Patient-reported quality of life is associated with severity of chronic graft-versus-host disease as measured by NIH criteria: report on baseline data from the Chronic GVHD Consortium. Blood. 2011;117:4651–7.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transpl. 2015;21:389–401.e381.
Kitko CL, White ES, Baird K. Fibrotic and sclerotic manifestations of chronic graft-versus-host disease. Biol Blood Marrow Transpl. 2012;18:S46–52.
MacDonald KP, Hill GR, Blazar BR. Chronic graft-versus-host disease: biological insights from preclinical and clinical studies. Blood. 2017;129:13–21.
Vadakkel G, Eng S, Proli A, Ponce DM. Updates in chronic graft-versus-host disease: novel treatments and best practices in the current era. Bone Marrow Transpl. 2024;59:1360–8.
Baird K, Steinberg SM, Grkovic L, Pulanic D, Cowen EW, Mitchell SA, et al. National Institutes of Health Chronic Graft-versus-Host Disease Staging in Severely Affected Patients: Organ and Global Scoring Correlate with Established Indicators of Disease Severity and Prognosis. Biol Blood Marrow Transpl. 2013;19:632–9.
Bolanos-Meade J, Hamadani M, Wu J, Al Malki MM, Martens MJ, Runaas L, et al. Post-Transplantation Cyclophosphamide-Based Graft-versus-Host Disease Prophylaxis. N Engl J Med. 2023;388:2338–48.
Arora M, Cutler CS, Jagasia MH, Pidala J, Chai X, Martin PJ, et al. Late Acute and Chronic Graft-versus-Host Disease after Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transpl. 2016;22:449–55.
Lee SJ, Wolff D, Kitko C, Koreth J, Inamoto Y, Jagasia M, et al. Measuring therapeutic response in chronic graft-versus-host disease. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. The 2014 Response Criteria Working Group report. Biol Blood Marrow Transpl. 2015;21:984–99.
Flowers MED, Martin PJ. How we treat chronic graft-versus-host disease. Blood. 2015;125:606–15.
Zanin-Zhorov A, Weiss JM, Nyuydzefe MS, Chen W, Scher JU, Depoil D, et al. Selective oral ROCK2 inhibitor down-regulates IL-21 and IL-17 secretion in human T cells via STAT3-dependent mechanism. Proc Natl Acad Sci USA. 2014;111:16814–9.
Flynn R, Paz K, Du J, Reichencbach DK, Taylor PA, Panoskaltsis-Mortari A, et al. Targeted Rho-associated kinase 2 inhibition suppresses murine and human chronic GVHD through a Stat3-dependent mechanism. Blood. 2016;127:2144–54.
Cutler C, Lee SJ, Arai S, Rotta M, Zoghi B, Lazaryan A, et al. Belumosudil for chronic graft-versus-host disease after 2 or more prior lines of therapy: the ROCKstar Study. Blood. 2021;138:2278–89.
Riches DW, Backos DS, Redente EF. ROCK and Rho: Promising therapeutic targets to ameliorate pulmonary fibrosis. Am J Pathol. 2015;185:909–12.
Przepiorka D, Le RQ, Ionan A, Lii EJ, Qang YH, Gudi R, et al. FDA Approval Summary: Belumosudil for Adult and Pediatric Patients 12 Years and Older with Chronic GvHD after Two or More Prior Lines of Systemic Therapy. Clin Cancer Res. 2022;28:2488–92.
Michonneau D, Malard F, Le Grand S, Magro L, D’Aveni M, Tudesq JJ, et al. Efficacy and safety of belumosudil for treatment of cGVHD: multicenter retrospective analysis of the French cohort of the compassionate use program, on behalf of the French Society of Bone Marrow Transplantation and Cellular Therapy. Bone Marrow Transpl. 2025;60:779–86.
Modi B, Ngo D, Chen J, Yang D, Shan H, Sandhu K, et al. Belumosudil for the treatment of chronic graft-versus-host disease: a single center real-world experience. Bone Marrow Transpl. 2025;60:555–7.
Heidenreich S, Egger-Heidrich K, Halter JP, Jost L, Stölzel F, Perl M, et al. Safety and efficacy of the ROCK-2-inhibitor Belumosudil in cGvHD treatment - a retrospective, German-Swiss multicenter real-world data analysis. Bone Marrow Transpl. 2025;60:439–46.
Chin MM, Tamaresis JS, Johnston LJ, Lowsky R, Meyer E, Muffly L, et al. Belumosudil combination therapy for chronic graft-versus-host-disease in real-world clinical practice. Bone Marrow Transpl. 2025;60:393–5.
Dierov D, Webb N, Fatmi S, Nwanne Cm Ciolino C, Mosesso K, et al. Establishing a standardized system for review and adjudication of chronic graft-vs-host disease data in accordance with the National Institutes Consensus criteria. Adv Cell Gene Ther. 2019;2:62.
Schoemans HM, Lee SJ, Ferrara JL, Wolff D, Levine JE, Schultz KR, et al. EBMT-NIH-CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transpl. 2018;53:1401–15.
Copelan E, Casper JT, Carter SL, van Burik JAH, Hurd D, Mendizabal AM, et al. A scheme for defining cause of death and its application in the T cell depletion trial. Biol Blood Marrow Transpl. 2007;13:1469–76.
Keever-Taylor C, Devine S, Soiffer R, Mendizabal A, Carter S, Pasquini MC, et al. Characteristics of CliniMACS® System CD34-enriched T cell-depleted grafts in a multicenter trial for acute myeloid leukemia-Blood and Marrow Transplant Clinical Trials Network (BMT CTN) protocol 0303. Biol Blood Marrow Transpl. 2012;18:690–7.
DeFilipp Z, Alousi AM, Pidala JA, Carpenter PA, Onstad LE, Arai S, et al. Nonrelapse mortality among patients diagnosed with chronic GVHD: an updated analysis from the Chronic GVHD Consortium. Blood Adv. 2021;5:4278–84.
Saidu NEB, Bonini C, Dickinson A, Grce M, Inngjerdingen M, Koehl U, et al. New Approaches for the Treatment of Chronic Graft-Versus-Host Disease: Current Status and Future Directions. Front Immunol. 2020;11:578314.
Malard F, Huang XJ, Sim JPY. Treatment and unmet needs in steroid-refractory acute graft-versus-host disease. Leukemia. 2020;34:1229–40.
Wolff D, Radojcic V, Lafyatis R, Cinar R, Rosenstein RK, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. The 2020 Highly morbid forms report. Transpl Cell Ther. 2021;27:817–35.
Lee SJ, Cutler C, Blazar BR, Tu A, Yang Z, Pavletic SZ. Correlation of Patient-Reported Outcomes with Clinical Organ Responses: Data from the Belumosudil Chronic Graft-versus-Host Disease Studies. Transpl Cell Ther. 2022;28:700 e701–700.e706.
Inamoto Y, Kato K, Kawakita T, Onishi Y, Matsuoka KI, Shiratori S, et al. An open-label study of belumosudil, a selective ROCK2 inhibitor, as second or subsequent line of therapy for steroid-dependent/steroid-resistant chronic GVHD. Am J Hematol. 2024;99:1917–26.
Acknowledgements
The work was also supported in part by the Memorial Sloan Kettering Cancer Center Support Grant (P30 CA008748); National Institutes of Health award number 5R01-HL164902-02.
Author information
Authors and Affiliations
Contributions
G.R., M.W., and D.M.P., designed the study, interpreted the data, and wrote the manuscript. D.N. and S.D. analyzed and interpreted the data and wrote the manuscript, I.G., A.S., P.M. collected the data, N.R. wrote the manuscript, M.A.P. interpreted the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
M.A.P reports honoraria from Adicet, Allogene, Allovir, Caribou Biosciences, Celgene, Bristol-Myers Squibb, Equilium, Exevir, ImmPACT Bio, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Nektar Therapeutics, Novartis, Omeros, OrcaBio, Sanofi, Syncopation, VectivBio AG, and Vor Biopharma. He serves on DSMBs for Cidara Therapeutics and Sellas Life Sciences, and the scientific advisory board of NexImmune. He has ownership interests in NexImmune, Omeros and OrcaBio. He has received institutional research support for clinical trials from Allogene, Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, and Novartis. D.M.P. has served as advisory board member for Evive Biotechnology (Shanghai) Ltd; has consulted, received honorarium from, or participated in the advisory boards for Sanofi, Ceramedix, and Incyte Corporation, and received research funding from Sanofi and Incyte. The other authors have no conflicts of interest to declare.
Ethics approval
All methods were performed in accordance with the relevant guidelines and regulations. This analysis was approved by the Memorial Sloan Kettering Cancer Center Institutional Review Board (IRB #16-501; characterization of graft-versus-host disease following hematopoietic stem cell transplantation). Written informed consent for participation was obtained from all study participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Raju, G., Walji, M., Nemirovsky, D. et al. Real-world experience of belumosudil and belumosudil/ruxolitinib combination in steroid-refractory chronic graft-versus-host disease. Bone Marrow Transplant (2025). https://doi.org/10.1038/s41409-025-02721-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41409-025-02721-z