Abstract
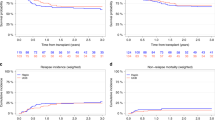
Choosing an optimal alternative donor is an important clinical concern in allogeneic hematopoietic cell transplantation (HCT). In Japan, single-unit umbilical cord blood transplantation (UCBT) has been widely used in the last two decades, whereas HCT from HLA-haploidentical related donors (haplo-HCT) has been increasingly used following the advent of posttransplant cyclophosphamide (PTCY) for graft-versus-host disease (GVHD) prophylaxis. This registry-based study aimed to compare outcomes between single-unit UCBT (n = 848) and PTCY-based haplo-HCT (n = 241) performed during first complete remission in patients with acute myeloid leukemia. UCBT was associated with a lower likelihood of engraftment (P < 0.001), a higher risk of grade 2–4 and grade 3–4 acute GVHD (P = 0.003 each), and a lower risk of extensive chronic GVHD (P = 0.048). The UCBT and haplo-HCT groups did not significantly differ in 3-year probabilities of overall survival (68% versus 69%, P = 0.686), GVHD/relapse-free survival (55% versus 54%, P = 0.866), relapse (14% versus 16%, P = 0.463), and non-relapse mortality (21% versus 19%, P = 0.403), respectively, which were confirmed with multivariate analysis. These results indicate that both procedures should be considered viable options for patients lacking a matched donor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data of this study are not publicly available due to ethical restrictions that it exceeds the scope of the recipient/donor’s consent for research use in the registry. However, data may be available from the corresponding author upon reasonable request and with permission of the JSTCT/JDCHCT.
References
Yanada M, Matsuo K, Emi N, Naoe T. Efficacy of allogeneic hematopoietic stem cell transplantation depends on cytogenetic risk for acute myeloid leukemia in first disease remission: a metaanalysis. Cancer. 2005;103:1652–8.
Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301:2349–61.
Yanada M. The evolving concept of indications for allogeneic hematopoietic cell transplantation during first complete remission of acute myeloid leukemia. Bone Marrow Transpl. 2021;56:1257–65.
Yanada M, Kurosawa S, Yamaguchi T, Uchida N, Miyawaki S, Kanamori H, et al. Effect of related donor availability on outcome of AML in the context of related and unrelated hematopoietic cell transplantation. Bone Marrow Transpl. 2013;48:390–5.
Labopin M, Gorin NC, Polge E, Socie G, Gurman G, Gluckman E, et al. A prospective registration study to determine feasibility of hematopoietic SCT in adults with acute leukemia: planning, expectations and reality. Bone Marrow Transpl. 2014;49:376–81.
Saito H, Ito M, Kato S, Kodera Y, Okamoto S, Taniguchi S, et al. The Japan Marrow Donor Program, 25 years of experience in achieving 20,000 bone marrow transplantations: organization structure, activity, and financial basis. Bone Marrow Transpl. 2018;53:609–16.
Dehn J, Chitphakdithai P, Shaw BE, McDonald AA, Devine SM, Burns LJ, et al. Likelihood of proceeding to allogeneic hematopoietic cell transplantation in the United States after search activation in the national registry: impact of patient age, disease, and search prognosis. Transplant Cell Ther. 2021;27:184 e1–84.e13.
Mawad R, Gooley TA, Sandhu V, Lionberger J, Scott B, Sandmaier BM, et al. Frequency of allogeneic hematopoietic cell transplantation among patients with high- or intermediate-risk acute myeloid leukemia in first complete remission. J Clin Oncol. 2013;31:3883–8.
Sugita J, Morita K, Konuma T, Yanada M. Allogeneic hematopoietic cell transplantation from alternative donors in acute myeloid leukemia. Ann Hematol. 2024;103:4851–68.
Yanada M, Takami A, Yamasaki S, Arai Y, Konuma T, Uchida N, et al. Allogeneic hematopoietic cell transplantation for adults with acute myeloid leukemia conducted in Japan during the past quarter century. Ann Hematol. 2020;99:1351–60.
Atsuta Y. Introduction of Transplant Registry Unified Management Program 2 (TRUMP2): scripts for TRUMP data analyses, part I (variables other than HLA-related data). Int J Hematol. 2016;103:3–10.
Yanada M, Konuma T, Kuwatsuka Y, Kondo T, Kawata T, Takahashi S, et al. Unit selection for umbilical cord blood transplantation for adults with acute myeloid leukemia in complete remission: a Japanese experience. Bone Marrow Transpl. 2019;54:1789–98.
Dohner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140:1345–77.
Holtan SG, DeFor TE, Lazaryan A, Bejanyan N, Arora M, Brunstein CG, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125:1333–8.
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9.
Yanada M, Mori J, Aoki J, Harada K, Mizuno S, Uchida N, et al. Effect of cytogenetic risk status on outcomes for patients with acute myeloid leukemia undergoing various types of allogeneic hematopoietic cell transplantation: an analysis of 7812 patients. Leuk Lymphoma. 2018;59:601–09.
Kharfan-Dabaja MA, Kumar A, Ayala E, Aljurf M, Nishihori T, Marsh R, et al. Standardizing definitions of hematopoietic recovery, graft rejection, graft failure, poor graft function, and donor chimerism in allogeneic hematopoietic cell transplantation: a report on Behalf of the American Society for Transplantation and Cellular Therapy. Transplant Cell Ther. 2021;27:642–49.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transpl. 1995;15:825–8.
Sullivan KM, Agura E, Anasetti C, Appelbaum F, Badger C, Bearman S, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28:250–9.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34:187–220.
Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706.
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509.
Fuchs EJ, O’Donnell PV, Eapen M, Logan B, Antin JH, Dawson P, et al. Double unrelated umbilical cord blood vs HLA-haploidentical bone marrow transplantation: the BMT CTN 1101 trial. Blood. 2021;137:420–28.
Sanz J, Montoro J, Solano C, Valcarcel D, Sampol A, Ferra C, et al. Prospective randomized study comparing myeloablative unrelated umbilical cord blood transplantation versus HLA-haploidentical related stem cell transplantation for adults with hematologic malignancies. Biol Blood Marrow Transpl. 2020;26:358–66.
El Jurdi N, Martens MJ, Brunstein CG, O’Donnell P, Lee SJ, D’Souza A, et al. Health-related quality of life in double umbilical cord blood versus haploidentical marrow transplantation: a quality of life analysis report of BMT CTN 1101. Transplant Cell Ther. 2023;29:467 e1–67.e5.
Ramsey SD, Bansal A, Li L, O’Donnell PV, Fuchs EJ, Brunstein CG, et al. Cost-effectiveness of unrelated umbilical cord blood transplantation versus HLA-haploidentical related bone marrow transplantation: evidence from BMT CTN 1101. Transplant Cell Ther. 2023;29:464 e1–64.e8.
Ruggeri A, Labopin M, Sanz G, Piemontese S, Arcese W, Bacigalupo A, et al. Comparison of outcomes after unrelated cord blood and unmanipulated haploidentical stem cell transplantation in adults with acute leukemia. Leukemia. 2015;29:1891–900.
Giannotti F, Labopin M, Shouval R, Sanz J, Arcese W, Angelucci E, et al. Haploidentical transplantation is associated with better overall survival when compared to single cord blood transplantation: an EBMT-Eurocord study of acute leukemia patients conditioned with thiotepa, busulfan, and fludarabine. J Hematol Oncol. 2018;11:110.
Ruggeri A, Labopin M, Savani B, Paviglianiti A, Blaise D, Volt F, et al. Hematopoietic stem cell transplantation with unrelated cord blood or haploidentical donor grafts in adult patients with secondary acute myeloid leukemia, a comparative study from Eurocord and the ALWP EBMT. Bone Marrow Transpl. 2019;54:1987–94.
Konuma T, Kanda J, Yamasaki S, Harada K, Shimomura Y, Terakura S, et al. Single cord blood transplantation versus unmanipulated haploidentical transplantation for adults with acute myeloid leukemia in complete remission. Transplant Cell Ther. 2021;27:334 e1–34.e11.
Ruggeri A, Galimard JE, Labopin M, Rafii H, Blaise D, Ciceri F, et al. Comparison of outcomes after unrelated double-unit cord blood and haploidentical peripheral blood stem cell transplantation in adults with acute myelogenous leukemia: a study on Behalf of Eurocord and the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Transplant Cell Ther. 2022;28:710 e1–10.e10.
Matsuda K, Konuma T, Fuse K, Masuko M, Kawamura K, Hirayama M, et al. Comparison of transplant outcomes between haploidentical transplantation and single cord blood transplantation in non-remission acute myeloid leukaemia: a nationwide retrospective study. Br J Haematol. 2023;201:106–13.
Murata M, Nakasone H, Kanda J, Nakane T, Furukawa T, Fukuda T, et al. Clinical factors predicting the response of acute graft-versus-host disease to corticosteroid therapy: an analysis from the GVHD Working Group of the Japan Society for Hematopoietic Cell Transplantation. Biol Blood Marrow Transpl. 2013;19:1183–9.
Metheny L, Politikos I, Ballen KK, Rezvani AR, Milano F, Barker JN, et al. Guidelines for adult patient selection and conditioning regimens in cord blood transplant recipients with hematologic malignancies and aplastic anemia. Transplant Cell Ther. 2021;27:286–91.
Ballen K, Woo Ahn K, Chen M, Abdel-Azim H, Ahmed I, Aljurf M, et al. Infection rates among acute leukemia patients receiving alternative donor hematopoietic cell transplantation. Biol Blood Marrow Transpl. 2016;22:1636–45.
Bejanyan N, Brunstein CG, Cao Q, Lazaryan A, Luo X, Curtsinger J, et al. Delayed immune reconstitution after allogeneic transplantation increases the risks of mortality and chronic GVHD. Blood Adv. 2018;2:909–22.
Brunstein CG, O’Donnell PV, Logan B, Dawson P, Costa L, Cutler C, et al. Impact of center experience with donor type on outcomes: a secondary analysis, blood and marrow transplant clinical trials network 1101Open for Accrual June 2012Open for Accrual June 2012. Transplant Cell Ther. 2022;28:406 e1–06.e6.
Yanada M, Yano S, Kuwatsuka Y, Kawamura K, Fukuda T, Ichinohe T, et al. The effect of center experience on allogeneic hematopoietic cell transplantation outcomes in acute myeloid leukemia. Bone Marrow Transpl. 2024;59:541–49.
Schmid C, Labopin M, Socie G, Daguindau E, Volin L, Huynh A, et al. Outcome of patients with distinct molecular genotypes and cytogenetically normal AML after allogeneic transplantation. Blood. 2015;126:2062–9.
Deol A, Sengsayadeth S, Ahn KW, Wang HL, Aljurf M, Antin JH, et al. Does FLT3 mutation impact survival after hematopoietic stem cell transplantation for acute myeloid leukemia? A Center for International Blood and Marrow Transplant Research (CIBMTR) analysis. Cancer. 2016;122:3005–14.
Jentzsch M, Bischof L, Ussmann J, Backhaus D, Brauer D, Metzeler KH, et al. Prognostic impact of the AML ELN2022 risk classification in patients undergoing allogeneic stem cell transplantation. Blood Cancer J. 2022;12:170.
Araki D, Wood BL, Othus M, Radich JP, Halpern AB, Zhou Y, et al. Allogeneic hematopoietic cell transplantation for acute myeloid leukemia: time to move toward a minimal residual disease-based definition of complete remission?. J Clin Oncol. 2016;34:329–36.
Oran B, Jorgensen JL, Marin D, Wang S, Ahmed S, Alousi AM, et al. Pre-transplantation minimal residual disease with cytogenetic and molecular diagnostic features improves risk stratification in acute myeloid leukemia. Haematologica. 2017;102:110–17.
Jentzsch M, Bischof L, Backhaus D, Brauer D, Schulz J, Franke GN, et al. Impact of MRD status in patients with AML undergoing allogeneic stem cell transplantation in the first vs the second remission. Blood Adv. 2022;6:4570–80.
Acknowledgements
This work was supported in part by the Grants-in-Aid for Scientific Research from JSPS KAKENHI (grant number: JP24K11566) (MY), and the Practical Research Project for Allergic Diseases and Immunology (Research Technology of Medical Transplantation) from the Japan Agency for Medical Research and Development (grant number: 19ek0510023h0003) (YA).
Author information
Authors and Affiliations
Contributions
MY designed the study, analyzed data, interpreted the results, and drafted the paper; SY, SM, JS, TF, YM, MT, NU, M Onizuka, ND, SO, MS, T Kawakita, YH, H Nakamae, KI, NH, H Nakasone, and T Konuma interpreted the results and edited the paper; and FI, JK, M Ohbiki, and YA contributed to data management, interpreted the results, and edited the paper. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Nagoya City University Institutional Review Board. All methods were performed in accordance with the Declaration of Helsinki, and all patients provided informed consent for the utilization of their clinical data for research.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yanada, M., Yamasaki, S., Mizuno, S. et al. Comparison of single-unit umbilical cord blood transplantation and haploidentical transplantation using posttransplant cyclophosphamide during first complete remission of acute myeloid leukemia. Bone Marrow Transplant 61, 18–25 (2026). https://doi.org/10.1038/s41409-025-02729-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41409-025-02729-5