Abstract
Objective The junctional epithelium (JE) is composed of tightly interconnected layers of squamous epithelial cells that play a crucial role as a defence mechanism. Recently, several enamel-derived proteins have been shown to play a role in the adhesion of JE cells to the mineralised tooth surface. This study aims to explore the in vivo protein expression levels of amelotin (AMTN), laminin (LAM332) and the protein secreted by follicular dendritic cells (FDC-SP) within the JE, both in the presence and absence of experimental periodontitis (EP) in rats.
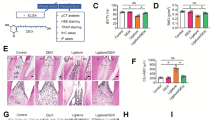
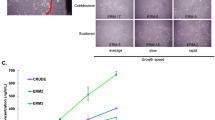
Materials and methods In total, 16 rats were randomly divided into two groups: a control group without EP and a group with induced periodontitis. EP was established by placing cotton ligatures around the cervical region of the lower first molars. Fifteen days after EP induction, all animals were euthanised and mandibles were harvested. Micro-computed tomography, histomorphometric, and immunohistochemistry analyses were employed to assess volumetric bone alterations, architectural bone parameters and number of osteoclasts. The presence of inflammatory cells and enamel protein expression were evaluated by immunofluorescence.
Results The results demonstrated that the EP group showed a significant increase in alveolar bone loss, an elevated number of tartrate-resistant acid phosphatase-positive cells, and enhanced inflammatory processes compared to the control group. Immunofluorescence staining revealed a significant increase in the expression of AMTN in the EP group. However, there were no significant differences between the expression of FDC-SP and LAM332. Correlation analysis of AMTN, LAM332 and FDC-SP with the intensity of inflammatory markers (CD45+, CD66b+ and CD8+, CD163+ and CD80+) showed no significant differences for the EP group.
Conclusion Our data suggest that the expression of AMTN increased after inflammatory stimuli and that AMTN may be associated with the onset and progression of EP.
Key points
-
It is essential to investigate the role of junctional epithelium proteins during periodontitis progression to understand their function under local inflammatory conditions. In this context, our study assessed the expression levels of enamel matrix proteins between healthy and diseased sites in the periodontium.
-
We showed that the localisation and expression of amelotin were significantly increased after inducing experimental periodontitis at the junctional epithelium. FDC-SP and LAM332 protein levels were not overexpressed during periodontitis progression. These findings suggest a potential role for amelotin in the inflammatory events that occur during the initiation and progression of periodontitis.
-
Increased protein expression of amelotin under inflammatory conditions underscores the role of amelotin as a diagnostic marker for disease onset/progression with direct clinical implications and paves the way for new approaches to managing periodontitis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The datasets used or analysed during the current study are available from the corresponding author upon reasonable request.
References
Li C, Gao Y, Xu Z et al. Expression and localization of amelotin, laminin gamma2 and odontogenesis-associated phosphoprotein (ODAPH) on the basal lamina and junctional epithelium. J Mol Histol 2022; 53: 111-118.
Bosshardt D D, Lang N P. The junctional epithelium: from health to disease. J Dent Res 2005; 84: 9-20.
Nouri S, Holcroft J, Caruso L-L et al. An SCPPPQ1/LAM332 protein complex enhances the adhesion and migration of oral epithelial cells: implications for dentogingival regeneration. Acta Biomater 2022; 147: 209-220.
Noguchi S, Ukai T, Kuramoto A et al. The histopathological comparison on the destruction of the periodontal tissue between normal junctional epithelium and long junctional epithelium. J Periodontal Res 2017; 52: 74-82.
Fischer N G, Aparicio C. Junctional epithelium and hemidesmosomes: tape and rivets for solving the ‘percutaneous device dilemma' in dental and other permanent implants. Bioact Mater 2022; 18: 178-198.
Iwasaki K, Bajenova E, Somogyi-Ganss E et al. Amelotin - a novel secreted, ameloblast-specific protein. J Dent Res 2005; 84: 1127-1132.
Moffatt P, Smith C E, St-Arnaud R, Simmons D, Wright J T, Nanci A. Cloning of rat amelotin and localization of the protein to the basal lamina of maturation stage ameloblasts and junctional epithelium. Biochem J 2006; 399: 37-46.
Ikeda Y, Neshatian M, Holcroft J, Ganss B. The enamel protein ODAM promotes mineralization in a collagen matrix. Connect Tissue Res 2018; 59: 62-66.
Fukumoto S, Kiba T, Hall B et al. Ameloblastin is a cell adhesion molecule required for maintaining the differentiation state of ameloblasts. J Cell Biol 2004; 167: 973-983.
Hu J C, Hu Y, Smith C E et al. Enamel defects and ameloblast-specific expression in Enam knock-out/lacz knock-in mice. J Biol Chem 2008; 283: 10858-10871.
Wright J T, Hart T C, Hart P S et al. Human and mouse enamel phenotypes resulting from mutation or altered expression of AMEL, ENAM, MMP20 and KLK4. Cells Tissues Organs 2009; 189: 224-229.
Gibson C W, Yuan Z A, Hall B et al. Amelogenin-deficient mice display an amelogenesis imperfecta phenotype. J Biol Chem 2001; 276: 31871-31875.
Nakayama Y, Inoue E, Kato A et al. Follicular dendritic cell-secreted protein gene expression is upregulated and spread in nifedipine-induced gingival overgrowth. Odontology 2020; 108: 532-544.
Nakayama Y, Kobayashi R, Matsui S et al. Localization and expression pattern of amelotin, odontogenic ameloblast-associated protein and follicular dendritic cell-secreted protein in the junctional epithelium of inflamed gingiva. Odontology 2017; 105: 329-337.
Lacruz R S, Nakayama Y, Holcroft J et al. Targeted overexpression of amelotin disrupts the microstructure of dental enamel. PLos One 2012; 7: e35200.
Nakayama Y, Holcroft J, Ganss B. Enamel hypomineralization and structural defects in amelotin-deficient mice. J Dent Res 2015; DOI: 10.1371/journal.pone.0035200.
Nakayama Y, Takai H, Matsui S et al. Proinflammatory cytokines induce amelotin transcription in human gingival fibroblasts. J Oral Sci 2014; 56: 261-268.
Nakayama Y, Takai H, Matsui S et al. Transcriptional regulation of amelotin gene by proinflammatory cytokines in gingival fibroblasts. Connect Tissue Res 2014; DOI: 10.3109/03008207.2014.923848.
Nakayama Y, Kobayashi R, Iwai Y et al. C/EBPbeta and YY1 bind and interact with Smad3 to modulate lipopolysaccharide-induced amelotin gene transcription in mouse gingival epithelial cells. FEBS Open Bio 2019; 9: 276-290.
Oshiro A, Iseki S, Miyauchi M et al. Lipopolysaccharide induces rapid loss of follicular dendritic cell-secreted protein in the junctional epithelium. J Periodontal Res 2012; 47: 689-694.
Takahashi S, Fukuda M, Mitani A et al. Follicular dendritic cell-secreted protein is decreased in experimental periodontitis concurrently with the increase of interleukin-17 expression and the Rankl/Opg mRNA ratio. J Periodontal Res 2014; 49: 390-397.
Marshall A J, Du Q, Draves K E, Shikishima Y, HayGlass K T, Clark E A. FDC-SP, a novel secreted protein expressed by follicular dendritic cells. J Immunol 2002; 169: 2381-2389.
Hormia M, Owaribe K, Virtanen I. The dento-epithelial junction: cell adhesion by type I hemidesmosomes in the absence of a true basal lamina. J Periodontol 2001; 72: 788-797.
Larjava H, Koivisto L, Hakkinen L, Heino J. Epithelial integrins with special reference to oral epithelia. J Dent Res 2011; 90: 1367-1376.
Ganss B, Abbarin N. Maturation and beyond: proteins in the developmental continuum from enamel epithelium to junctional epithelium. Front Physiol 2014; 5: 371.
Kinumatsu T, Hashimoto S, Muramatsu T et al. Involvement of laminin and integrins in adhesion and migration of junctional epithelium cells. J Periodontal Res 2009; 44: 13-20.
Fouillen A, Dos Santos Neves J, Mary C et al. Interactions of AMTN, ODAM and SCPPPQ1 proteins of a specialized basal lamina that attaches epithelial cells to tooth mineral. Sci Rep 2017; 7: 46683.
Percie du Sert N, Hurst V, Ahluwalia A et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLos Biol 2020; DOI: 10.1371/journal.pbio.3000410.
Ervolino E, Statkievicz C, Toro L F et al. Antimicrobial photodynamic therapy improves the alveolar repair process and prevents the occurrence of osteonecrosis of the jaws after tooth extraction in senile rats treated with zoledronate. Bone 2019; 120: 101-113.
Garcia V G, Longo M, Gualberto Júnior E C et al. Effect of the concentration of phenothiazine photosensitizers in antimicrobial photodynamic therapy on bone loss and the immune inflammatory response of induced periodontitis in rats. J Periodontal Res 2014; 49: 584-594.
Nogueira A V B, Nokhbehsaim M, Tekin S et al. Resistin is increased in periodontal cells and tissues: in vitro and in vivo studies. Mediators Inflamm 2020; 2020: 9817095.
De Molon R S, Hsu C, Bezouglaia O et al. Rheumatoid arthritis exacerbates the severity of osteonecrosis of the jaws (onj) in mice. a randomized, prospective, controlled animal study. J Bone Miner Res 2016; 31: 1596-1607.
De Molon R S, Shimamoto H, Bezouglaia O et al. OPG-Fc but not zoledronic acid discontinuation reverses osteonecrosis of the jaws (ONJ) in mice. J Bone Miner Res 2015; 30: 1627-1640.
Fernandes N A R, Camilli A C, Maldonado L A G et al. Chalcone T4, a novel chalconic compound, inhibits inflammatory bone resorption in vivo and suppresses osteoclastogenesis in vitro. J Periodontal Res 2021; 56: 569-578.
Nogueira A V, de Souza J A, de Molon R S et al. HMGB1 localization during experimental periodontitis. Mediators Inflamm 2014; 2014: 816320.
Lopes M E S, Marcantonio C C, de Molon R S et al. Obesity influences the proteome of periodontal ligament tissues following periodontitis induction in rats. J Periodontal Res 2022; 57: 545-557.
Gomes N A, Guarenghi G G, Valenga H M et al. Mandibular-related bone metabolism in orchiectomized rats treated with sex hormones. Arch Oral Biol 2021; 122: 105000.
Costes S V, Daelemans D, Cho E H, Dobbin Z, Pavlakis G, Lockett S. Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys J 2004; 86: 3993-4003.
Nishio C, Wazen R, Moffatt P, Nanci A. Expression of odontogenic ameloblast-associated and amelotin proteins in the junctional epithelium. Periodontol 2000 2013; 63: 59-66.
Nakamura S, Terashima T, Yoshida T et al. Identification of genes preferentially expressed in periodontal ligament: specific expression of a novel secreted protein, FDC-SP. Biochem Biophys Res Commun 2005; 338: 1197-1203.
Shinomura T, Nakamura S, Ito K, Shirasawa S, Höök M, Kimura J H. Adsorption of follicular dendritic cell-secreted protein (FDC-SP) onto mineral deposits. Application of a new stable gene expression system. J Biol Chem 2008; 283: 33658-33664.
De Molon R S, Mascarenhas V I, de Avila E D et al. Long-term evaluation of oral gavage with periodontopathogens or ligature induction of experimental periodontal disease in mice. Clin Oral Investig 2016; 20: 1203-1216.
De Molon R S, de Avila E D, Boas Nogueira A V et al. Evaluation of the host response in various models of induced periodontal disease in mice. J Periodontol 2014; 85: 465-477.
De Molon R S, de Avila E D, Cirelli J A. Host responses induced by different animal models of periodontal disease: a literature review. J Investig Clin Dent 2013; 4: 211-218.
Acknowledgements
The authors express their special thanks to the staff at the Faculty of Dentistry, University of Toronto, for the extensive help during immunofluorescence processing.
Funding
Rafael Scaf de Molon is currently supported by The Sao Paulo Research Foundation (Fundacao de Amparo a Pesquisa do Estado de Sao Paulo) FAPESP grant #2023/15750-7. The authors acknowledge the financial support provided by the Brazilian funding agency coordination for the improvement of higher education (CAPES), financial code 01 in the scope of CAPES/print - funding code: 001 (process: 88887.194785/2018-00).
Author information
Authors and Affiliations
Contributions
Conceptualisation: VGG, RSdM, LHT; methodology: NAG, VGG, GMS, E E, TER, BG, AA, MM, RSdM, LHT; validation: NAG, VGG, GMS, E E, TER, BG, AA, MM, RSdM, LHT; formal analysis: NAG, VGG, GMS, E E, TER, BG, AA, MM, RSdM, LHT; investigation: NAG, VGG, GMS, RSdM, LHT; data curation: NAG, VGG, GMS, E E, TER, BG, AA, MM, RSdM, LHT; writing - original draft preparation, RSdM; writing - review and editing: NAG, VGG, GMS, E E, TER, BG, AA, MM, RSdM, LHT; visualisation: NAG, VGG, GMS, E E, TER, BG, AA, MM, RSdM, LHT; supervision: VGG and LHT. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
The authors declare no conflicts of interest. This study was performed in line with the principles of the ARRIVE guidelines. The approval for the study was granted by the Animal Use Ethics Committee (#00608-2020) from the Sao Paulo State University, UNESP, School of Dentistry at Aracatuba.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gomes, N., Garcia, V., Mulinari-Santos, G. et al. Expression and localisation of amelotin, laminin and protein secreted by follicular dendritic cells after ligature-induced experimental periodontitis in rats. Br Dent J (2025). https://doi.org/10.1038/s41415-025-8508-7
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41415-025-8508-7