Abstract
Background Remimazolam is a novel ultra-short-acting benzodiazepine which shows a high efficacy and safety profile for medical procedures, such as endoscopy; however, its use in dentistry is relatively new and lacks an evidence base.
Aims The service evaluation aimed to establish the safety and efficacy of remimazolam as a conscious sedation agent in dental outpatient settings since its introduction into two secondary care sedation and special care dental services in 2023.
Method Retrospective data collection of remimazolam sedation cases from April 2023 to April 2024 was carried out.
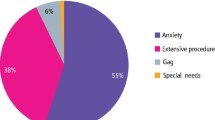
Results The success rate of 111 remimazolam sedation cases was 97%. The mean interval between sedation start time and beginning of treatment was 2-4 minutes, while the mean time from the last administered dose to patient discharge was 24 minutes. The mean dose of remimazolam administered was 11.9 mg, with a range of 1.5-35 mg. Patients required a mean of four additional doses, with a mean interval of 5.7 minutes between top-ups. The majority of cases (82.9%) resulted in Ellis scores of 1 or 2. Five complications were documented: three cases of desaturation, one case of disinhibition and one case of post-operative hypertension, none of which necessitated flumazenil reversal.
Conclusion This service evaluation provided promising insights into the potential safety profile and effectiveness of remimazolam for dental sedation in two secondary care settings; however, larger-scale studies are necessary to substantiate these findings. This is a rapidly emerging area of sedation practice that is likely to see an influx of research and development in the near future.
Key points
-
Remimazolam - a novel ultra-short-acting benzodiazepine with its quick onset and offset - showed enhanced safety and efficacy in endoscopy which could be used in dentistry, particularly in medically complex patients.
-
With careful case selection, remimazolam can facilitate successful completion of a range of dental procedures safely.
-
The incidence of failures and complications was low; however, further larger-scale studies in a range of dental settings are required to validate its effectiveness and safety profile.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Access to anonymised data may be considered on a case-by-case basis, subject to institutional approval and appropriate data-sharing agreements. Requests for data should be directed to the corresponding author and will be reviewed in compliance with Trust guidelines and patient confidentiality policies.
References
Royal College of Surgeons. Remimazolam for Intravenous Conscious Sedation for Dental Procedures: IACSD Standard on Clinical Use and Training. 2023. Available at https://www.rcseng.ac.uk/-/media/FDS/IACSD/IACSD-remimazolam-Statement-130623.pdf (accessed 1 September 2023).
Ul-Haque I, Shaikh T G, Ahmed S H et al. Efficacy of remimazolam for procedural sedation in American Society of Anesthesiologists (ASA) i to iv patients undergoing colonoscopy: a systematic review and meta-analysis. Cureus 2022; DOI: 10.7759/cureus.22881.
Goudra B G, Singh P M. Remimazolam: the future of its sedative potential. Saudi J Anaesth 2014; 8: 388-391.
Noor N, Legendre R, Cloutet A, Chitneni A, Varrassi G, Kaye A D. A comprehensive review of remimazolam for sedation. Health Psychol Res 2021; 9: 24514.
Oka S, Satomi H, Sekino R et al. Sedation outcomes for remimazolam, a new benzodiazepine. J Oral Sci 2021; 63: 209-211.
Zhu X, Wang H, Yuan S et al. Efficacy and safety of remimazolam in endoscopic sedation - a systematic review and meta-analysis. Front Med 2021; 8: 655042.
Guo Z, Wang X, Wang L, Liu Y, Yang X. Can remimazolam be a new sedative option for outpatients undergoing ambulatory oral and maxillofacial surgery? J Oral Maxillofac Surg 2023; 81: 8-16.
Li X, Tian M, Deng Y, She T, Li K. Advantages of sedation with remimazolam compared to midazolam for the removal of impacted tooth in patients with dental anxiety. J Oral Maxillofac Surg 2023; 81: 536-545.
Ba K, Ni D, Du R, Wei X. Advantages of remimazolam for sedation in impacted tooth extraction. Hua Xi Kou Qiang Yi Xue Za Zhi 2024; 42: 476-480.
Swart R, Maes S S A Cavanaugh D, Mason K P. Remimazolam pilot for office-based dental sedation: adverse events, awareness and outcomes. J Clin Med 2023; 12: 7308.
Oue K, Oda A, Shimizu Y et al. Efficacy and safety of remimazolam besilate for sedation in outpatients undergoing impacted third molar extraction: a prospective exploratory study. BMC Oral Health 2023; 23: 774.
European Medicines Agency. Remimazolam product information: Annex 1, summary of product characteristics. 2021. Available at https://www.ema.europa.eu/en/documents/product-information/byfavo-epar-product-information_en.pdf (accessed 1 September 2023).
Ellis S. Response to intravenous midazolam sedation in general dental practice. Br Dent J 1996; 180: 417-420.
UK Government. Draft Misuse of Drugs Act 1971 (Amendment) Order 2024. 2024. Available at https://hansard.parliament.uk/Commons/2024-01-24/debates/f0c637a9-3869-4b74-95db-567910d19db6/DraftMisuse OfDrugsAct1971%28Amendment%29Order2024 (accessed 1 December 2024).
Kim K M. Remimazolam: pharmacological characteristics and clinical applications in anesthesiology. Anesth Pain Med 2022; 17: 1-11.
Rex D K, Bhandari R, Desta T et al. A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest Endosc 2018; 88: 427-437.
Doshi M, Prasad R, Reilly D. A service evaluation of the use of IV sedation with remimazolam for adults with an acquired brain injury. SAAD Dig 2025: 41: 22-27.
Liow A M, Akuffo N, Yeo X H, Clough S. Remimazolam as a sedative for dental procedures: a case series. SAAD Dig 2025: 41: 28-31.
Dao V-A, Schippers F, Stöhr T. Efficacy of remimazolam versus midazolam for procedural sedation: post hoc integrated analyses of three phase 3 clinical trials. Endosc Int Open 2022; DOI: 10.1055/a-1743-1936.
Rex D K, Bhandari R, Lorch D G, Meyers M, Schippers F, Bernstein D. Safety and efficacy of remimazolam in high risk colonoscopy:a randomized trial. Dig Liver Dis 2021; 53: 94-101.
Pastis N J, Yarmus L B, Schippers F et al. Safety and efficacy of remimazolam compared with placebo and midazolam for moderate sedation during bronchoscopy. Chest 2019; 155: 137-146.
Acknowledgements
The authors would like to thank all the sedationists involved in data collection and the special care dentistry team and the pharmacy team who contributed to the proposal leading to the approval of remimazolam use in the respective services.
Author information
Authors and Affiliations
Contributions
XHY: study design, data collection, data analysis, interpretation of results and manuscript preparation. MD: data collection, review of results and data analysis, manuscript review. SC: study proposal and design, supervision of data collection, review of results and data analysis, manuscript review. ZS: study proposal and design, data collection, review of results and data analysis, manuscript review. The final manuscript was reviewed and finalised by all authors.
Corresponding author
Ethics declarations
The authors declare there are no conflicts of interest. This is a retrospective service evaluation and ethics approval was not required according to the Medical Research Council HRA toolkit. The retrospective nature of data collection meant that it had no influence on patient care/interventions. Consent was not required. Under NHS guidelines, all patients are informed that their data may be used for audit and monitoring purposes unless they choose to opt out. Additionally, all patients provided written consent for the use of remimazolam as part of their treatment plan. Both services have registered the service evaluation with their respective audit/clinical effectiveness unit (CEU).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yeo, X., Doshi, M., Clough, S. et al. A multi-site service evaluation on remimazolam for dental conscious sedation. Br Dent J (2025). https://doi.org/10.1038/s41415-025-8717-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41415-025-8717-0