Abstract
Background
FOLFIRINOX has shown promising results in locally advanced (LAPA) or borderline resectable (BRPA) pancreatic adenocarcinoma. We report here a cohort of patients treated with this regimen from the AGEO group.
Methods
This is a retrospective multicentre study. We included all consecutive patients with non-pre-treated LAPA or BRPA treated with FOLFIRINOX.
Results
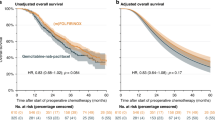
We included 330 patients (57.9% male, 65.4% <65 years, 96.4% PS <2). Disease was classified as BRPA in 31.1% or LAPA in 68.9%. Objective response rate with FOLFIRINOX was 29.5% and stable disease 51%. Subsequent CRT was performed in 46.4% of patients and 23.9% had curative intent surgery. Resection rates were 42.1% for BRPA and 15.5% for LAPA. Main G3/4 toxicities were fatigue (15%), neutropenia (12%) and neuropathy (G2/3 35%). After a median follow-up of 26.7 months, median OS (mOS) and PFS were 21.4 and 12.4 months, respectively. For patients treated by FOLFIRINOX alone, or FOLFIRINOX followed by CRT, or FOLFIRINOX + /− CRT + surgery, mOS was 16.8 months, 21.8 months and not reached, respectively (p < 0.0001).
Conclusions
FOLFIRINOX for LAPA and BRPA seems to be effective with a manageable toxicity profile. These promising results in “real-life” patients now have to be confirmed in a Phase 3 randomised trial.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 68, 7–30 (2018).
Institut National du Cancer. Les cancers en France en 2015, l’essentiel des faits et chiffres. http://www.e-cancer.fr/Expertises-et-publications/Catalogue-des-publications/Les-cancers-en-France-en-2015-L-essentiel-des-faits-et-chiffres (2016).
Stathis, A. & Moore, M. J. Advanced pancreatic carcinoma: current treatment and future challenges. Nat. Rev. Clin. Oncol. 7, 163–172 (2010).
Ducreux, M., Cuhna, A. S., Caramella, C., Hollebecque, A., Burtin, P., Goéré, D. et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 26, v56–v68 (2015).
Katz, M. H. G., Marsh, R., Herman, J. M., Shi, Q., Collison, E., Venook, A. P. et al. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann. Surg. Oncol. 20, 2787–2795 (2013).
Tempero, M. A., Malafa, M. P., Chiorean, E. G., Czito, B., Scaife, C., Narang, A. K. et al. Pancreatic Adenocarcinoma, Version 1.2019. J. Natl Compr. Cancer Netw. 17, 202–210 (2019).
Neoptolemos, J. P., Palmer, D. H., Ghaneh, P., Psarelli, E. E., Valle, J. W., Halloran, C. M. et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 389, 1011–1024 (2017).
Suker, M., Beumer, B. R., Sadot, E., Marthey, L., Faris, J. E., Mellon, E. A. et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. 17, 801–810 (2016).
Chauffert, B., Mornex, F., Bonnetain, F., Rougier, P., Mariette, C., Bouché, O. et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann. Oncol. 19, 1592–1599 (2008).
Petrelli, F., Coinu, A., Borgonovo, K., Cabiddu, M., Ghilardi, M., Lonati, V. et al. FOLFIRINOX-based neoadjuvant therapy in borderline resectable or unresectable pancreatic cancer: a meta-analytical review of published studies. Pancreas 44, 515–521 (2015).
Versteijne, E., Suker, M., Groothuis, K., Akkermans-Vogelaar, J. M., Besselink, M. G., Bonsing, B. A. et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the Dutch randomized phase III PREOPANC trial. J. Clin. Oncol. 38, 1763–1773 (2020).
Conroy, T., Desseigne, F., Ychou, M., Bouché, O., Guimbaud, R., Bécouarn, Y. et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 364, 1817–1825 (2011).
Conroy, T., Hammel, P., Hebbar, M., Ben Abdelghani, M., Wei, A. C., Raoul, J.-L. et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N. Engl. J. Med. 379, 2395–2406 (2018).
Marthey, L., Sa-Cunha, A., Blanc, J. F., Gauthier, M., Cueff, A., Francois, E. et al. FOLFIRINOX for locally advanced pancreatic adenocarcinoma: results of an AGEO multicenter prospective observational cohort. Ann. Surg. Oncol. 22, 295–301 (2015).
Hosein, P. J., Macintyre, J., Kawamura, C., Maldonado, J. C., Ernani, V., Loaiza-Bonilla, A. et al. A retrospective study of neoadjuvant FOLFIRINOX in unresectable or borderline-resectable locally advanced pancreatic adenocarcinoma. BMC Cancer 12, 199 (2012).
Katz, M. H. G., Shi, Q., Ahmad, S. A., Herman, J. M., Marsh, R., de, W., Collisson, E. et al. Preoperative modified FOLFIRINOX treatment followed by capecitabine-based chemoradiation for borderline resectable pancreatic cancer: alliance for clinical trials in oncology trial A021101. JAMA Surg. 151, e161137 (2016).
Murphy, J. E., Wo, J. Y., Ryan, D. P., Jiang, W., Yeap, B. Y., Drapek, L. C. et al. Total neoadjuvant therapy with FOLFIRINOX followed by individualized chemoradiotherapy for borderline resectable pancreatic adenocarcinoma: a phase 2 clinical trial. JAMA Oncol. 4, 963–969 (2018).
Maggino, L., Malleo, G., Marchegiani, G., Viviani, E., Nessi, C., Ciprani, D. et al. Outcomes of primary chemotherapy for borderline resectable and locally advanced pancreatic ductal adenocarcinoma. JAMA Surg. 154, 932–942 (2019).
Conroy, T., Paillot, B., François, E., Bugat, R., Jacob, J.-H., Stein, U. et al. Irinotecan plus oxaliplatin and leucovorin-modulated fluorouracil in advanced pancreatic cancer-a Groupe Tumeurs Digestives of the Federation Nationale des Centres de Lutte Contre le Cancer study. J. Clin. Oncol. 23, 1228–1236 (2005).
Faris, J. E., Blaszkowsky, L. S., McDermott, S., Guimaraes, A. R., Szymonifka, J., Huynh, M. A. et al. FOLFIRINOX in locally advanced pancreatic cancer: the Massachusetts General Hospital Cancer Center experience. Oncologist 18, 543–548 (2013).
Gunturu, K. S., Yao, X., Cong, X., Thumar, J. R., Hochster, H. S., Stein, S. M. et al. FOLFIRINOX for locally advanced and metastatic pancreatic cancer: single institution retrospective review of efficacy and toxicity. Med. Oncol. 30, 361 (2013).
Hammel, P., Huguet, F., van Laethem, J.-L., Goldstein, D., Glimelius, B., Artru, P. et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA 315, 1844–1853 (2016).
Trakul, N., Koong, A. C. & Chang, D. T. Stereotactic body radiotherapy in the treatment of pancreatic cancer. Semin. Radiat. Oncol. 24, 140–147 (2014).
Herman, J. M., Chang, D. T., Goodman, K. A., Dholakia, A. S., Raman, S. P., Hacker-Prietz, A. et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer 121, 1128–1137 (2015).
Chuong, M. D., Springett, G. M., Freilich, J. M., Park, C. K., Weber, J. M., Mellon, E. A. et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int. J. Radiat. Oncol. Biol. Phys. 86, 516–522 (2013).
Palta, M., Czito, B. G., Duffy, E., Malicki, M., Niedzwiecki, D., Abbruzzese, J. et al. A phase II trial of neoadjuvant gemcitabine/nab-paclitaxel and SBRT for potentially resectable pancreas cancer: an evaluation of acute toxicity. J. Clin. Oncol. 36, 4121 (2018).
Quan, K., Sutera, P., Xu, K., Bernard, M. E., Burton, S. A., Wegner, R. E. et al. Results of a prospective phase 2 clinical trial of induction gemcitabine/capecitabine followed by stereotactic ablative radiation therapy in borderline resectable or locally advanced pancreatic adenocarcinoma. Pract. Radiat. Oncol. 8, 95–106 (2018).
Acknowledgements
The authors would like to acknowledge David Marsh (language review).
Author information
Authors and Affiliations
Contributions
E.A.: study design, statistical analysis, manuscript writing and editing. L. Marthey: study design, data collection, manuscript writing, R.A., L. Mas, E. Francois, A.S., A.S.C., A.V., T.L., V.H., C.d.L.F., M.S., F.K., J.F., R.C., E. Fabiano, F.L., N.W.: data collection, manuscript writing, J.B.B., D.T.: study design, manuscript writing, J.T.: study design, data analysis, manuscript writing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No informed consent was needed for this observational study, as stated by the French ethics committee consulted prior to the beginning of the work.
Data availability
Data are available from J.T. at reasonable request.
Competing interests
E.A.: Travel expenses: Mundipharma. Lectures and educational activities: Sanofi Genzymes, Lilly-Oncology. J.T.: Consulting or advisory role: Roche, Merck KGaA, Darmstadt, Germany, Amgen, Celgene, Eli Lilly, Servier, Sirtex Medical, Merck Sharp & Dohme, Pierre Fabre. Speakers’ Bureau: Servier, Amgen, Roche/Genentech, Sanofi, Merck KGaA, Darmstadt, Germany, Eli Lilly, Merck Sharp & Dohme, Pierre Fabre. The other authors have nothing to declare.
Funding information
This study was not funded.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Auclin, E., Marthey, L., Abdallah, R. et al. Role of FOLFIRINOX and chemoradiotherapy in locally advanced and borderline resectable pancreatic adenocarcinoma: update of the AGEO cohort. Br J Cancer 124, 1941–1948 (2021). https://doi.org/10.1038/s41416-021-01341-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41416-021-01341-w
This article is cited by
-
Neoadjuvantes FOLFIRINOX vs. neoadjuvante Radiochemotherapie und adjuvante Chemotherapie mit Gemcitabin für resektable und borderline-resektable Pankreaskarzinome (PREOPANC-2-Studie)
Strahlentherapie und Onkologie (2026)
-
Preoperative chemotherapy, radiotherapy and surgical decision-making in patients with borderline resectable and locally advanced pancreatic cancer
Nature Reviews Gastroenterology & Hepatology (2024)
-
Irreversible electroporation to bring initially unresectable locally advanced pancreatic adenocarcinoma to surgery: the IRECAP phase II study
European Radiology (2024)
-
Proton radiotherapy as a treatment strategy to increase survival in locally advanced pancreatic cancer in the body and tail: a retrospective study
Radiation Oncology (2023)