Fig. 1: Targeting cancer neoantigens using cell therapy.
From: Promises and challenges of adoptive T-cell therapies for solid tumours
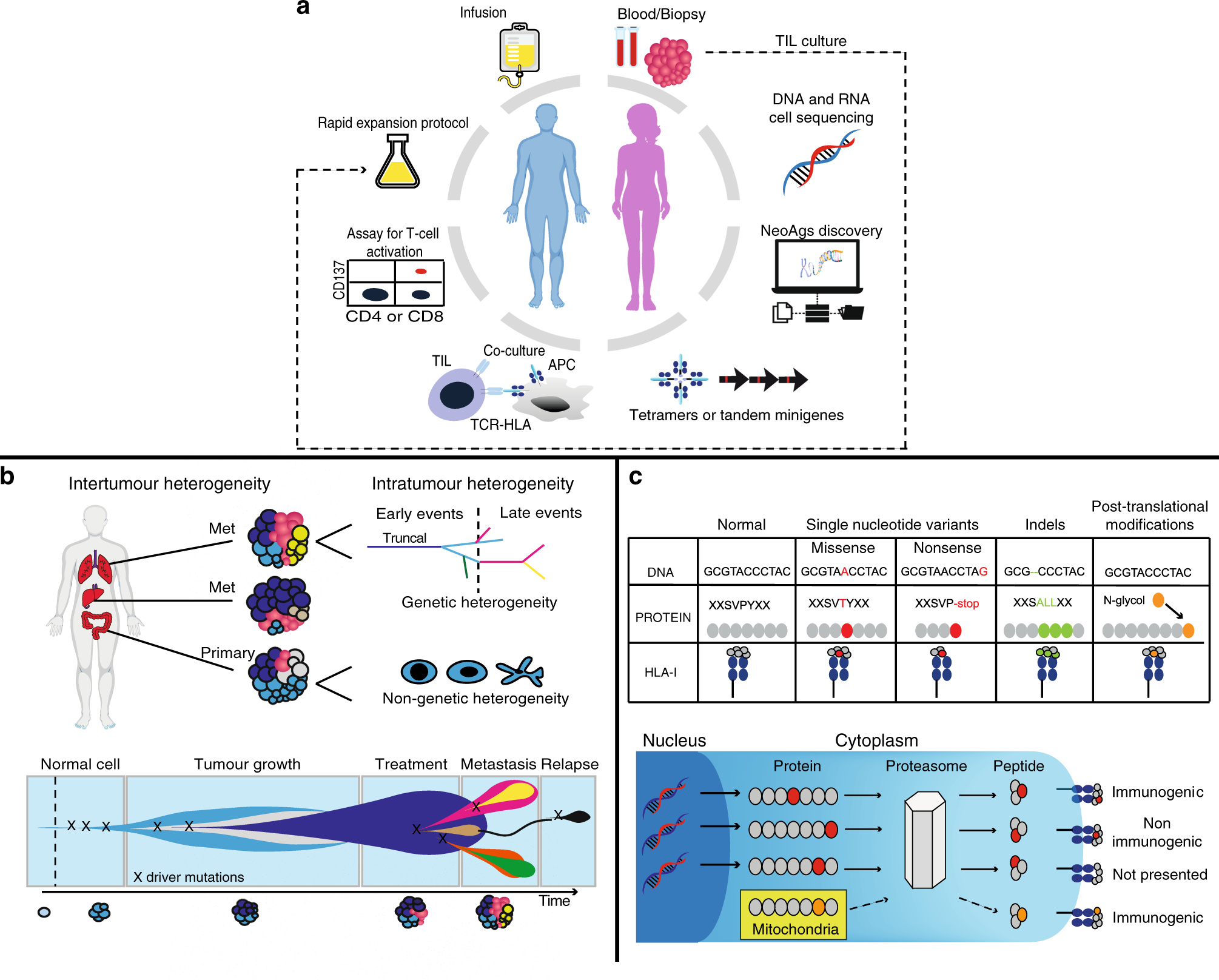
a Using autologous tumour-infiltrating lymphocytes in autologous cell transfer. The resected specimen is divided into multiple tumour fragments that are individually grown in IL-2 for 7–10 days. For the ‘non-specific’ TIL therapy (dashed line) the individual cultures are then moved to a rapid expansion protocol before reinfusion into patients. Neoantigen-TIL therapy involves the sequencing of exomic or whole-genome DNA from tumour cells and healthy cells to identify tumour-specific mutations, before RNA-sequencing is used to check for the expression of mutations. Corresponding minigenes or peptides encoding each mutated amino acid are synthesised and expressed in or pulsed into a patient’s autologous antigen-presenting cells (APCs) for presentation in the context of a patient’s HLA. Individual mutations responsible for tumour recognition are identified by analysing activation of a T-cell co-stimulatory marker, such as 41BB/CD137 (CD8+ T cells), in response to cognate target antigen recognition. b Genetic and genomic heterogeneity and evolution of clonal populations. Upper panel: Genetic and phenotypic variations are observed between tumours of different tissues (inter-tumour heterogeneity). Within a tumour, subclonal diversity can be observed (intra-tumour heterogeneity, different colours of tumour clones). Clonal alterations occurring early in tumorigenesis are represented by the blue trunk of the phylogenetic tree (truncal mutations); later alterations could be shared by tumour cells in some regions of the tumour (light blue and pink branches of the tree) or present in only one region of the tumour (yellow branches of the tree) in a branched cancer evolution model. Tumour subclones can also show differential gene expression due to non-genetic heterogeneity. Lower row: Unique clones (represented by different colours) emerge as a consequence of accumulating driver mutations in the progeny of a single most recent common ancestor cell. Ongoing linear and branching evolution results in multiple simultaneous subclones that can individually give rise to episodes of disease relapse and metastasis. c Overview of the neoantigen landscape. The sources of potential neoantigens for HLA class I ligands are shown. In tumours, mutated or aberrantly expressed proteins are processed via the proteasome into peptides. The cross-priming abilities of peptides are also linked to non-genetic factors such as protein stability, which can be modulated by several factors, including their localisation in the mitochondria. These peptides can be loaded onto HLA class I molecules and might or might not elicit a CD8+ T-cell response, depending on several factors, including peptide sequence or T-cell receptor (TCR) sequences. In general, most of the neoantigens derived from single-nucleotide variants gain their immunogenicity through altered amino acids involved in direct T-cell contact.
