Abstract
Purpose
PARP inhibitor resistance may be overcome by combinatorial strategies with agents that disrupt homologous recombination repair (HRR). Multiple HRR pathway components are HSP90 clients, so that HSP90 inhibition leads to abrogation of HRR and sensitisation to PARP inhibition. We performed in vivo preclinical studies of the HSP90 inhibitor onalespib with olaparib and conducted a Phase 1 combination study.
Patients and methods
Tolerability and efficacy studies were performed in patient-derived xenograft(PDX) models of ovarian cancer. Clinical safety, tolerability, steady-state pharmacokinetics and preliminary efficacy of olaparib and onalespib were evaluated using a standard 3 + 3 dose-escalation design.
Results
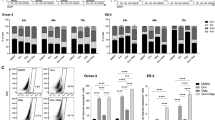
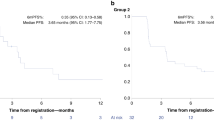
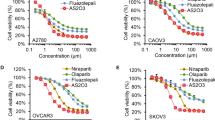
Olaparib/onalespib exhibited anti-tumour activity against BRCA1-mutated PDX models with acquired PARPi resistance and PDX models with RB-pathway alterations(CDKN2A loss and CCNE1 overexpression). Phase 1 evaluation revealed that dose levels up to olaparib 300 mg/onalespib 40 mg and olaparib 200 mg/onalespib 80 mg were safe without dose-limiting toxicities. Coadministration of olaparib and onalespib did not appear to affect the steady-state pharmacokinetics of either agent. There were no objective responses, but disease stabilisation ≥24 weeks was observed in 7/22 (32%) evaluable patients including patients with BRCA-mutated ovarian cancers and acquired PARPi resistance and patients with tumours harbouring RB-pathway alterations.
Conclusions
Combining onalespib and olaparib was feasible and demonstrated preliminary evidence of anti-tumour activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Konstantinopoulos PA, Ceccaldi R, Shapiro GI, D’Andrea AD. Homologous recombination deficiency: exploiting the fundamental vulnerability of ovarian cancer. Cancer Discov. 2015;5:1137–54.
Konstantinopoulos PA, Barry WT, Birrer M, Westin SN, Cadoo KA, Shapiro GI, et al. Olaparib and alpha-specific PI3K inhibitor alpelisib for patients with epithelial ovarian cancer: a dose-escalation and dose-expansion phase 1b trial. Lancet Oncol. 2019;20:570–80.
Veneris JT, Matulonis UA, Liu JF, Konstantinopoulos PA. Choosing wisely: selecting PARP inhibitor combinations to promote anti-tumor immune responses beyond BRCA mutations. Gynecol Oncol. 2020;156:488–97.
Barrott JJ, Haystead TA. Hsp90, an unlikely ally in the war on cancer. FEBS J. 2013;280:1381–96.
Pearl LH. Hsp90 and Cdc37—a chaperone cancer conspiracy. Curr Opin Genet Dev. 2005;15:55–61.
Pearl LH, Prodromou C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu Rev Biochem. 2006;75:271–94.
Konstantinopoulos PA, Papavassiliou AG. 17-AAG: mechanisms of antitumour activity. Expert Opin Investig Drugs. 2005;14:1471–4.
Pennisi R, Ascenzi P, di Masi A. Hsp90: a new player in DNA repair? Biomolecules. 2015;5:2589–618.
Stecklein SR, Kumaraswamy E, Behbod F, Wang W, Chaguturu V, Harlan-Williams LM, et al. BRCA1 and HSP90 cooperate in homologous and non-homologous DNA double-strand-break repair and G2/M checkpoint activation. Proc Natl Acad Sci USA. 2012;109:13650–5.
Choi YE, Battelli C, Watson J, Liu J, Curtis J, Morse AN, et al. Sublethal concentrations of 17-AAG suppress homologous recombination DNA repair and enhance sensitivity to carboplatin and olaparib in HR proficient ovarian cancer cells. Oncotarget. 2014;5:2678–87.
Johnson N, Johnson SF, Yao W, Li YC, Choi YE, Bernhardy AJ, et al. Stabilization of mutant BRCA1 protein confers PARP inhibitor and platinum resistance. Proc Natl Acad Sci USA. 2013;110:17041–6.
Do K, Speranza G, Chang LC, Polley EC, Bishop R, Zhu W, et al. Phase I study of the heat shock protein 90 (Hsp90) inhibitor onalespib (AT13387) administered on a daily for 2 consecutive days per week dosing schedule in patients with advanced solid tumors. Invest N Drugs. 2015;33:921–30.
Lomeli N, Bota DA. Targeting HSP90 in malignant gliomas: onalespib as a potential therapeutic. Transl Cancer Res. 2018;7:6215–26.
Canella A, Welker AM, Yoo JY, Xu J, Abas FS, Kesanakurti D, et al. Efficacy of onalespib, a long-acting second-generation HSP90 inhibitor, as a single agent and in combination with temozolomide against malignant gliomas. Clin Cancer Res. 2017;23:6215–26.
Slovin S, Hussain S, Saad F, Garcia J, Picus J, Ferraldeschi R, et al. Pharmacodynamic and clinical results from a phase I/II study of the HSP90 inhibitor onalespib in combination with abiraterone acetate in prostate cancer. Clin Cancer Res. 2019;25:4624–33.
Liu JF, Palakurthi S, Zeng Q, Zhou S, Ivanova E, Huang W, et al. Establishment of patient-derived tumor xenograft models of epithelial ovarian cancer for pre-clinical evaluation of novel therapeutics. Clin Cancer Res. 2016; https://doi.org/10.1158/1078-0432.CCR-16-1237.
Kochupurakkal BS, Parmar K, Lazaro J-B, Unitt C, Zeng Q, Reavis H, et al. Abstract 2796: development of a RAD51-based assay for determining homologous recombination proficiency and PARP inhibitor sensitivity. Cancer Res. 2017;77:2796–2796.
Parmar K, Kochupurakkal BS, Lazaro JB, Wang ZC, Palakurthi S, Kirschmeier PT, et al. The CHK1 inhibitor prexasertib exhibits monotherapy activity in high-grade serous ovarian cancer models and sensitizes to PARP inhibition. Clin Cancer Res. 2019;25:6127–40.
Liu JF, Palakurthi S, Zeng Q, Zhou S, Ivanova E, Huang W, et al. Establishment of patient-derived tumor xenograft models of epithelial ovarian cancer for preclinical evaluation of novel therapeutics. Clin Cancer Res. 2017;23:1263–73.
Shapiro GI, Kwak E, Dezube BJ, Yule M, Ayrton J, Lyons J, et al. First-in-human phase I dose escalation study of a second-generation non-ansamycin HSP90 inhibitor, AT13387, in patients with advanced solid tumors. Clin Cancer Res. 2015;21:87–97.
Curry J, Fazal L, Graham B, Harada I, Lyons J, Reule M, et al. Significance of long term pharmacodynamic actions of the HSP90 inhibitor AT13387. Cancer Res. 2009;69:1856–1856.
Shimamura T, Perera SA, Foley KP, Sang J, Rodig SJ, Inoue T, et al. Ganetespib (STA-9090), a nongeldanamycin HSP90 inhibitor, has potent antitumor activity in in vitro and in vivo models of non-small cell lung cancer. Clin Cancer Res. 2012;18:4973–85.
Sholl LM, Do K, Shivdasani P, Cerami E, Dubuc AM, Kuo FC, et al. Institutional implementation of clinical tumor profiling on an unselected cancer population. JCI insight. 2016;1:e87062.
Wagle N, Berger MF, Davis MJ, Blumenstiel B, Defelice M, Pochanard P, et al. High-throughput detection of actionable genomic alterations in clinical tumor samples by targeted, massively parallel sequencing. Cancer Discov. 2012;2:82–93.
Garcia EP, Minkovsky A, Jia Y, Ducar MD, Shivdasani P, Gong X, et al. Validation of OncoPanel: a targeted next-generation sequencing assay for the detection of somatic variants in cancer. Arch Pathol Lab Med. 2017;141:751–8.
Lindemann K, Kristensen G, Mirza MR, Davies L, Hilpert F, Romero I, et al. Poor concordance between CA-125 and RECIST at the time of disease progression in patients with platinum-resistant ovarian cancer: analysis of the AURELIA trial. Ann Oncol. 2016;27:1505–10.
Dean E, Middleton MR, Pwint T, Swaisland H, Carmichael J, Goodege-Kunwar P, et al. Phase I study to assess the safety and tolerability of olaparib in combination with bevacizumab in patients with advanced solid tumours. Br J Cancer. 2012;106:468–74.
Cancer Genome Atlas Research, N. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15.
Gorski JW, Ueland FR, Kolesar JM. CCNE1 Amplification as a predictive biomarker of chemotherapy resistance in epithelial ovarian cancer. Diagnostics. 2020;10:279.
Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–12.
Bedin M, Gaben AM, Saucier C, Mester J. Geldanamycin, an inhibitor of the chaperone activity of HSP90, induces MAPK-independent cell cycle arrest. Int J Cancer. 2004;109:643–52.
Burrows F, Zhang H, Kamal A. Hsp90 activation and cell cycle regulation. Cell Cycle. 2004;3:1530–6.
Etemadmoghadam D, Weir BA, Au-Yeung G, Alsop K, Mitchell G, George J, et al. Synthetic lethality between CCNE1 amplification and loss of BRCA1. Proc Natl Acad Sci USA. 2013;110:19489–94.
Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer. 2011;11:558–72.
Hallett ST, Pastok MW, Morgan RML, Wittner A, Blundell K, Felletar I, et al. Differential regulation of G1 CDK complexes by the Hsp90-Cdc37 chaperone system. Cell Rep. 2017;21:1386–98.
Lamphere L, Fiore F, Xu X, Brizuela L, Keezer S, Sardet C, et al. Interaction between Cdc37 and Cdk4 in human cells. Oncogene. 1997;14:1999–2004.
Vaughan CK, Gohlke U, Sobott F, Good VM, Ali MM, Prodromou C, et al. Structure of an Hsp90-Cdc37-Cdk4 complex. Mol Cell. 2006;23:697–707.
Garsed DW, Alsop K, Fereday S, Emmanuel C, Kennedy CJ, Etemadmoghadam D, et al. Homologous recombination DNA repair pathway disruption and retinoblastoma protein loss are associated with exceptional survival in high-grade serous ovarian cancer. Clin Cancer Res. 2018;24:569–80.
Ohkubo S, Kodama Y, Muraoka H, Hitotsumachi H, Yoshimura C, Kitade M, et al. TAS-116, a highly selective inhibitor of heat shock protein 90alpha and beta, demonstrates potent antitumor activity and minimal ocular toxicity in preclinical models. Mol Cancer Ther. 2015;14:14–22.
Polier S, Samant RS, Clarke PA, Workman P, Prodromou C, Pearl LH. ATP-competitive inhibitors block protein kinase recruitment to the Hsp90-Cdc37 system. Nat Chem Biol. 2013;9:307–12.
Siddiqui FA, Parkkola H, Manoharan GB, Abankwa D. Medium-throughput detection of Hsp90/Cdc37 protein-protein interaction inhibitors using a split Renilla luciferase-based assay. SLAS Discov. 2020;25:195–206.
Siddiqui FA, Parkkola H, Vukic V, Oetken-Lindholm C, Jaiswal A, Kiriazis A, et al. Novel small molecule Hsp90/Cdc37 interface inhibitors indirectly target K-Ras-signaling. Cancers. 2021;13:927.
Zhang Q, Wu X, Zhou J, Zhang L, Xu X, Zhang L, et al. Design, synthesis and bioevaluation of inhibitors targeting HSP90-CDC37 protein-protein interaction based on a hydrophobic core. Eur J Med Chem. 2021;210:112959.
Acknowledgements
We thank all the patients and their families for participation in the present trial.
Funding
The preclinical work was supported in part by the Department of Defense Ovarian Cancer Research Program Award DOD W81XWH-15-0564/OC140632 (PAK, Principal Investigator). The trial (clinicalTrials.gov Identifier: NCT02898207) was sponsored by the NCI-Cancer Therapy Evaluation Program (CTEP) and conducted under the auspices of the Experimental Therapeutics Clinical Trials Network (ETCTN). Dana-Farber/Harvard Cancer Center served as the lead site for the study, supported by NIH grant UM1 CA186709 (GIS, Principal Investigator). SPI is an employee of NIH and a member of NCI-CTEP as Associate Chief of the Investigational Drug Branch and Program Director of the ETCTN.
Author information
Authors and Affiliations
Contributions
PAK conceived, supervised, coordinated, and analysed all the data and wrote the manuscript; S-CC performed the statistical analysis and was the lead statistician of the study; JGS performed the PK analyses; MP, BB, HS, PB, MH and JC contributed to data collection and coordinated the processing and distribution of the clinical trial samples; AEW, PI, NH, AAW and SMC provided clinical data and contributed to the analysis of data; EVI, CPP, SP, JFL, ADD and DC performed and contributed to the preclinical studies and to the analysis of data; UAM and GIS supported and supervised the analyses of this study. All authors contributed to the writing and editing of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The clinical trial was approved by the NCI Central Institutional Review Board (CIRB) and the US Food and Drug Administration (NCT02898207). All procedures involving human participants were carried out in accordance with the Declaration of Helsinki. Written informed consent was obtained from patients or guardians before enrolment in the study.
Consent to publish
Not applicable.
Competing interests
Dr. Konstantinopoulos has participated in advisory boards for Alkermes, AstraZeneca, GSK, BMS, Repare, Artios and Kadmon, serves in the gynaecologic oncology scientific committee for AstraZeneca and has received institutional support as P.I. of clinical trials from AstraZeneca, Bayer, Eli Lilly, GSK, Merck, Merck KGaA, Pfizer and GSK. Dr. Cloud Paweletz has sponsored research agreements with Daiichi Sankyo, Bicycle Therapeutics, Transcenta, Bicara Therapeutics, AstraZeneca, Intellia Therapeutics, Janssen Pharmaceuticals, Array Biopharma, has stock and other ownership Interests in XSphera Biosciences, has received honoraria from Bio-Rad and ThermoFisher and has consulting or advisory role for DropWorks and XSphera Biosciences. Dr. Liu has participated in advisory boards from AstraZeneca, Clovis, Eisai, EpsilaBio, Genentech, GSK/Tesaro, Regeneron Pharmaceuticals and has received institutional support as P.I. of clinical trials from 2X Oncology, Aravive, Arch Oncology, AstraZeneca, Bristol-Myers Squibb, Clovis Oncology, CytomX Therapeutics, GlaxoSmithKline, Regeneron, Surface Oncology, Tesaro, Vigeo therapeutics. Dr. Campos has participated in the advisory board for AstraZeneca. Dr. Wright has participated in the advisory board for GSK. Dr. Matulonis has participated in advisory boards for Novartis, Blueprint Medicines, Trillium, in data safety monitoring boards for Advaxis and Symphogen and has been a consultant for NextCure, Astrazeneca and Merck. Dr. D’Andrea is a consultant/advisory board member for Lilly Oncology, Merck-EMD-Serono, Intellia Therapeutics, Sierra Oncology, Cyteir Therapeutics, Third Rock Ventures, AstraZeneca, Ideaya Inc., Cedilla Therapeutics Inc., a stockholder in Ideaya Inc., Cedilla Therapeutics Inc., and Cyteir, and reports receiving commercial research grants from Lilly Oncology and Merck-EMD-Serono. Dr. Shapiro has received research funding from Eli Lilly, Merck KGaA/EMD-Serono, Merck, and Sierra Oncology, has served on advisory boards for Pfizer, Eli Lilly, G1 Therapeutics, Roche, Merck KGaA/EMD-Serono, Sierra Oncology, Bicycle Therapeutics, Fusion Pharmaceuticals, Cybrexa Therapeutics, Astex, Almac, Ipsen, Bayer, Angiex, Daiichi Sankyo, Seattle Genetics, Boehringer Ingelheim, ImmunoMet, Asana, Artios, Atrin, Concarlo Holdings, Syros, Zentalis, CytomX Therapeutics and Blueprint Medicines and holds a patent entitled, “Dosage regimen for sapacitabine and seliciclib,” also issued to Cyclacel Pharmaceuticals, and a pending patent, entitled, “Compositions and Methods for Predicting Response and Resistance to CDK4/6 Inhibition,” together with Liam Cornell.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Konstantinopoulos, P.A., Cheng, SC., Supko, J.G. et al. Combined PARP and HSP90 inhibition: preclinical and Phase 1 evaluation in patients with advanced solid tumours. Br J Cancer 126, 1027–1036 (2022). https://doi.org/10.1038/s41416-021-01664-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41416-021-01664-8
This article is cited by
-
Stability-indicating UPLC method for quantification of alpelisib in bulk and tablet formulation by QbD approach
Future Journal of Pharmaceutical Sciences (2025)
-
Hyperthermia-induced BRCA2 reduction potentiates PARPi sensitivity in BRCA2-proficient ovarian carcinoma
Discover Oncology (2025)
-
Heat shock proteins as hallmarks of cancer: insights from molecular mechanisms to therapeutic strategies
Journal of Hematology & Oncology (2024)
-
Combination therapy involving HSP90 inhibitors for combating cancer: an overview of clinical and preclinical progress
Archives of Pharmacal Research (2024)
-
New ruthenium-xanthoxylin complex eliminates colorectal cancer stem cells by targeting the heat shock protein 90 chaperone
Cell Death & Disease (2023)