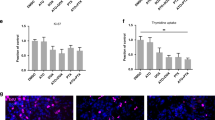
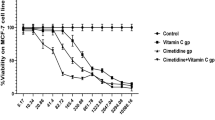
Abstract
Background
Targeted cancer therapy is an alternative to standard chemotherapy for a better prognosis. Although its incompetency for triple-negative breast cancer (TNBC), treatment still relies on classical chemotherapy. Increasing evidence suggest that chemotherapeutic drug-induced toxic effect could be minimised by combinatorial therapy. Profilin’s familiar anti-tumorigenic activity can be utilised in combination with the drug to improve efficacy, which could be promising therapeutics to treat TNBC.
Methods
All-trans retinoic acid (ATRA) in combination with vinblastine was tested on human MDA MB-231 cell line (MB-231) (in vitro) and MB-231 borne breast cancer in nude mice (in vivo). Effects of combination treatment on tumour growth inhibition and apoptosis were examined by tumour volume, histology and PARP cleavage. ATRA-induced transcriptional regulation of profilin had been evaluated by gel-shift and reporter gene assays. Profilin’s role in ATRA-induced vinblastine efficacy was validated in profilin-stable and profilin-silenced cells.
Results
ATRA binds with RAR/RXR to increase the profilin expression that potentiated cell death by chemotherapeutics. ATRA priming led to vinblastine-mediated potentiation of G2–M phase cell cycle arrest in MB-231 cells and regression of breast cancer in xenograft mice at very low concentration without any adverse effects. Moreover, increased p53 and PTEN but downregulated p65 in the tumour tissues further supported the involvement of profilin for tumour regression.
Conclusions
Vinblastine at very low concentration (20 times lesser than the recommended dose for breast cancer therapeutic) significantly regress tumour growth in ATRA-primed mice without any toxic effects suggesting potential combinatorial therapeutics for TNBC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Carlsson L, Nystrom LE, Sundkvist I, Markey F, Lindberg U. Actin polymerizability is influenced by Profilin, a low molecular weight protein in non-muscle cells. J Mol Biol. 1977;115:465–483.
Ding Z, Gau D, Deasy B, Wells A, Roy P. Both actin and polyproline interactions of Profilin-1 are required for migration, invasion and capillary morphogenesis of vascular endothelial cells. Exp Cell Res. 2009;315:2963–2973.
Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465.
Ding Z, Lambrechts A, Parepally M, Roy P. Silencing Profilin-1 inhibits endothelial cell proliferation, migration and cord morphogenesis. J Cell Sci. 2006;119:4127–4137.
Zaidi AH, Manna SK. Profilin–PTEN interaction suppresses NF-κB activation via inhibition of IKK phosphorylation. Biochem J. 2016;473:859–872.
Ding Z, Joy M, Bhargava R, Gunsaulus M, Lakshman N, Miron-Mendoza M, et al. Profilin-1 downregulation has contrasting effects on early vs late steps of breast cancer metastasis. Oncogene. 2014;33:2065–2074.
Choi YN, Lee SK, Seo TW, Lee JS, Yoo SJ. C-Terminus of Hsc70-interacting protein regulates Profilin1 and breast cancer cell migration. Biochem Biophys Res Commun. 2014;446:1060–1066.
Witke W, Sutherland JD, Sharpe A, Arai M, Kwiatkowski DJ. Profilin I is essential for cell survival and cell division in early mouse development. PNAS. 2001;98:3832–3836.
Witke W. The role of Profilin complexes in cell motility and other cellular processes. Trends Cell Biol. 2004;14:461–69.
Jiang C, Ding Z, Joy M, Chakraborty S, Kim SH, Bottcher R, et al. A balanced level of profilin-1 promotes stemness and tumor-initiating potential of breast cancer cells. Cell Cycle. 2017;16:2366–2373.
Wendell KL, Wilson L, Jordan MA. Mitotic block in HeLa cells by vinblastine: ultrastructural changes in kinetochore-mcirotubule attachment and in centrosomes. J Cell Sci. 1993;104:261–274.
Chemocare. Vinblastine. 2021. http://chemocare.com/chemotherapy/drug-info/Vinblastine.aspx.
Schinzari G, Rossi E, Cassano A, Dadduzio V, Quirino M, Pagliara M, et al. Cisplatin, dacarbazine and vinblastine as first line chemotherapy for liver metastatic uveal melanoma in the era of immunotherapy: a single institution phase II study. Melanoma Res. 2017;17 27:591–595.
Ahmed T, Yagoda A, Needles B, Scher HI, Watson RC, Geller N. Vinblastine and methotrexate for advanced bladder cancer. J Urol. 1985;133:602–604.
Garewal HS, Brooks RJ, Jones SE, Miller TP. Treatment of advanced breast cancer with mitomycin C combined with vinblastine or vindesine. J Clin Oncol. 1983;12:772–775.
Roma-Rodrigues C, Rivas-García L, Baptista PV, Fernandes AR. Gene therapy in cancer treatment: why go nano? Pharmaceutics. 2020;12:233.
Wu N, Zhang W, Yang Y, Liang Y, Wang L, Jin J, et al. Profilin 1 obtained by proteomic analysis in a 11-trans retinoic acid-treated hepatocarcinoma cell lines is involved in inhibition of cell proliferation and migration. Proteomics. 2006;6:6095–6106.
Zaidi AH, Raviprakash N, Mokhamatam RB, Gupta P, Manna SK. Profilin potentiates chemotherapeutic agents mediated cell death via suppression of NF-κB and upregulation of p53. Apoptosis. 2016;21:502–513.
Li Z, Zhong Q, Yang T, Xie X, Chen M. The role of profilin-1 in endothelial cell injury induced by advanced glycation end products (AGEs). Cardiovasc Diabetol. 2013;12:141.
Matijević T, Pavelić J. Poly(I:C) treatment influences the expression of calreticulin and profilin-1 in a human HNSCC cell line: a proteomic study. Tumour Biol. 2012;33:1201–1208.
Luo T, Sakai Y, Wagner E, Drager UC. Retinoids, eye development, and maturation of visual function. J Neurobiol. 2006;66:677–686.
Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res. 2002;43:1773–1808.
Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol. 2006;66:606–630.
Long Q, Zhou M, Liu X, Du Y, Fan J, Li X, et al. Interaction of CCN1 with αvβ3 integrin induces P-glycoprotein and confers vinblastine resistance in renal cell carcinoma cells. Anti Cancer Drugs. 2013;24:810–817.
Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, et al. Erratum: Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2006;116:2827.
Yap HY, Blumenschein GR, Keating MJ, Hortobagyi GN, Tashima CK, Loo TL. Vinblastine given as a continuous 5-day infusion in the treatment of refractory advanced breast cancer. Cancer Treat Rep. 1980;64:279–283.
Kaushik A, Kelsoe G, Jaton JC. The nude mutation results in impaired primary antibody repertoire. Eur J Immunol. 1995;25:631–634.
Price JE. Metastasis from human breast cancer cell lines. Breast cancer Res Treat. 1996;39:93–102.
Raviprakash N, Manna SK. Short-term exposure to oleandrin enhances responses to IL-8 by increasing cell surface IL-8 receptors. Br J Pharmacol. 2014;171:3339–3351.
Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63.
Mohun AF, Cook IJY. Simple methods for measuring serum levels of the glutamic-oxalacetic and glutamic- pyruvic transaminases in routine laboratories. J Clin Pathol. 1957;10:394–399.
Patrad E, Niapour A, Farassati F, Amani M. Combination treatment of all-trans retinoic acid (ATRA) and γ-secretase inhibitor (DAPT) cause growth inhibition and apoptosis induction in the human gastric cancer cell line. Cytotechnology. 2018;70:865–877.
Schenk T, Stengel S, Zelent A. Unlocking the potential of retinoic acid in anticancer therapy. Br J Cancer. 2014;111:2039–2045.
Gene Transcription Regulation Database. V20.06, as on 1st June 2019. http://gtrd.biouml.org/.
Rastinejad F, Wagner T, Zhao Q, Khorasanizadeh S. Structure of the RXR-RAR DNA-binding complex on the retinoic acid response element DR1. EMBO J. 2000;19:1045–1054.
Price JE, Polyzos A, Zhang RD, Daniels LM. Tumorigenicity and metastasis of human breast carcinoma cell Lines in nude mice. Cancer Res. 1990;50:717–721.
Liu Y, Chou C, Kim M, Vasisht R, Kuo Y, Ang P, et al. Assessing metastatic potential of breast cancer cells based on EGFR dynamics. Sci Rep. 2019;9:3395.
Janke J, Schlüter K, Jandrig B, Theile M, Kölble K, Arnold W, et al. Suppression of tumorigenicity in breast cancer cells by the microfilament protein profilin 1. J Exp Med. 2000;191:1675–1685.
Frantzi M, Klimou Z, Makridakis M, Zoidakis J, Latosinska A, Borràs DM, et al. Silencing of Profilin-1 suppresses cell adhesion and tumor growth via predicted alterations in integrin and Ca2+ signaling in T24M-based bladder cancer models. Oncotarget. 2016;7:70750–70768.
Shen K, Xi Z, Xie J, Wang H, Xie C, Lee CS, et al. Guttiferone K suppresses cell motility and metastasis of hepatocellular carcinoma by restoring aberrantly reduced profilin 1. Oncotarget. 2016;7:56650–56663.
Wei J, Ye C, Liu F, Wang W. All-trans retinoic acid and arsenic trioxide induce apoptosis and modulate intracellular concentrations of calcium in hepatocellular carcinoma cells. J Chemother. 2014;26:348–352.
Arce F, Gätjens-Boniche O, Vargas E, Valverde B, Díaz C. Apoptotic events induced by naturally occurring retinoids ATRA and 13-cis retinoic acid on human hepatoma cell lines Hep3B and HepG2. Cancer Lett. 2005;229:271–281.
Aebi S, Kröning R, Cenni B, Sharma A, Fink D, Los G, et al. all-trans retinoic acid enhances cisplatin-induced apoptosis in human ovarian adenocarcinoma and in squamous head and neck cancer cells. Clin Cancer Res. 1997;11:2033–2038.
Orfali N, O’Donovan TR, Cahill MR, Benjamin D, Nanus DM, McKenna SL, et al. All-trans retinoic acid (ATRA)-induced TFEB expression is required for myeloid differentiation in acute promyelocytic leukemia (APL). Eur J Haematol. 2020;104:236–250.
Jiang C, Veon W, Li H, Hallows KR, Roy P. Epithelial morphological reversion drives Profilin-1-induced elevation of p27kip1 in mesenchymal triple-negative human breast cancer cells through AMP-activated protein kinase activation. Cell Cycle. 2015;14:2914–2923.
Zhu C, Kim SJ, Mooradian A, Wang F, Li Z, Holohan S, et al. Cancer-associated exportin-6 upregulation inhibits the transcriptionally repressive and anticancer effects of nuclear profilin-1. Cell Rep. 2021;34:108749.
Mills KI, Walsh V, Gilkes AF, Woodgate LJ, Brown G, Burnett AK. Identification of transcription factors expressed during ATRA-induced neutrophil differentiation of HL60 cells. Br J Haematol. 1998;103:87–92.
Acknowledgements
We thank CDFDs’ Animal House Facility and Sophisticated Equipment Facility (SEF) for their support in this study.
Funding
This work was partially supported by the core grant of Centre for DNA Fingerprinting and Diagnostics (CDFD). We thank Department of Biotechnology (DBT), Govt. of India for providing fellowship to SS and the fund from DBT-NER grant.
Author information
Authors and Affiliations
Contributions
SS performed all the experiments, data curation, investigation, analysis, manuscript writing and SKM conceptualised the project, supervised and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Saurav, S., Manna, S.K. Increased expression of Profilin potentiates chemotherapeutic agent-mediated tumour regression. Br J Cancer 126, 1410–1420 (2022). https://doi.org/10.1038/s41416-021-01683-5
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41416-021-01683-5