Abstract
Background
Recent data suggest that BRAFV600E-mutated metastatic colorectal cancer (mCRC) patients with right-sided tumours and ECOG-PS = 0 may achieve benefit from the triplet regimen differently than those with left-sided tumours and ECOG-PS > 0.
Methods
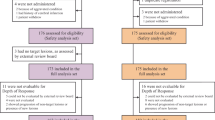
The predictive impact of primary sidedness and ECOG-PS was evaluated in a large real-life dataset of 296 BRAFV600E-mutated mCRC patients treated with upfront triplet or doublet ± bevacizumab. Biological differences between right- and left-sided BRAFV600E-mutated CRCs were further investigated in an independent cohort of 1162 samples.
Results
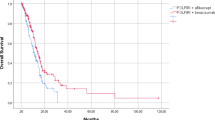
A significant interaction effect between primary sidedness and treatment intensity was reported in terms of both PFS (p = 0.010) and OS (p = 0.003), with a beneficial effect of the triplet in the right-sided group and a possible detrimental effect in the left-sided. No interaction effect was observed between ECOG-PS and chemo-backbone. In the MSS/pMMR population, a consistent trend for a side-related subgroup effect was observed when FOLFOXIRI ± bevacizumab was compared to oxaliplatin-based doublets±bevacizumab (p = 0.097 and 0.16 for PFS and OS, respectively). Among MSS/pMMR tumours, the BM1 subtype was more prevalent in the right-sided group (p = 0.0019, q = 0.0139). No significant differences were observed according to sidedness in the MSI-H/dMMR population.
Conclusions
Real-life data support the use of FOLFOXIRI ± bevacizumab only in BRAFV600E-mutated mCRC patients with right-sided tumours.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Datasets supporting the results of this work are available to editors, referees and readers promptly upon request.
References
Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. https://doi.org/10.1038/nature00766
Richman SD, Seymour MT, Chambers P, Elliott F, Daly CL, Meade AM, et al. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC FOCUS trial. J Clin Oncol. 2009;27:5931–7. https://doi.org/10.1200/JCO.2009.22.4295
Tran B, Kopetz S, Tie J, Gibbs P, Jiang Z-Q, Lieu CH, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011;117:4623–32. https://doi.org/10.1002/cncr.26086
Morris V, Overman MJ, Jiang Z-Q, Garrett C, Agarwal S, Eng C, et al. Progression-free survival remains poor over sequential lines of systemic therapy in patients with BRAF-mutated colorectal cancer. Clin Colorectal Cancer. 2014;13:164–71. https://doi.org/10.1016/j.clcc.2014.06.001
Seligmann JF, Fisher D, Smith CG, Richman SD, Elliott F, Brown S, et al. Investigating the poor outcomes of BRAF-mutant advanced colorectal cancer: analysis from 2530 patients in randomised clinical trials. Ann Oncol. 2017;28:562–8. https://doi.org/10.1093/annonc/mdw645
Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–422. https://doi.org/10.1093/annonc/mdw235
Loupakis F, Cremolini C, Masi G, Lonardi S, Zagonel V, Salvatore L, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371:1609–18. https://doi.org/10.1056/NEJMoa1403108
Cremolini C, Loupakis F, Antoniotti C, Lupi C, Sensi E, Lonardi S, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16:1306–15. https://doi.org/10.1016/S1470-2045(15)00122-9
Cremolini C, Antoniotti C, Rossini D, Lonardi S, Loupakis F, Pietrantonio F, et al. Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020;21:497–507. https://doi.org/10.1016/S1470-2045(19)30862-9
Cremolini C, Antoniotti C, Stein A, Bendell J, Gruenberger T, Rossini D, et al. Individual Patient Data Meta-Analysis of FOLFOXIRI Plus Bevacizumab Versus Doublets Plus Bevacizumab as Initial Therapy of Unresectable Metastatic Colorectal Cancer. J Clin Oncol. 2020. https://doi.org/10.1200/JCO.20.01225
Moretto R, Giordano M, Poma AM, Passardi A, Boccaccino A, Pietrantonio F, et al. Exploring clinical and gene expression markers of benefit from FOLFOXIRI/bevacizumab in patients with BRAF-mutated metastatic colorectal cancer: subgroup analyses of the TRIBE2 study. Eur J Cancer. 2021;153:16–26. https://doi.org/10.1016/j.ejca.2021.04.039
Arnold D, Lueza B, Douillard J-Y, Peeters M, Lenz H-J, Venook A, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. 2017;28:1713–29. https://doi.org/10.1093/annonc/mdx175
Holch JW, Ricard I, Stintzing S, Modest DP, Heinemann V. The relevance of primary tumour location in patients with metastatic colorectal cancer: a meta-analysis of first-line clinical trials. Eur J Cancer. 2017;70:87–98. https://doi.org/10.1016/j.ejca.2016.10.007
Cremolini C, Morano F, Moretto R, Berenato R, Tamborini E, Perrone F, et al. Negative hyper-selection of metastatic colorectal cancer patients for anti-EGFR monoclonal antibodies: the PRESSING case-control study. Ann Oncol. 2017;28:3009–14. https://doi.org/10.1093/annonc/mdx546
Morano F, Corallo S, Lonardi S, Raimondi A, Cremolini C, Rimassa L, et al. Negative hyperselection of patients with RAS and BRAF wild-type metastatic colorectal cancer who received panitumumab-based maintenance therapy. J Clin Oncol. 2019;37:3099–110. https://doi.org/10.1200/JCO.19.01254
Loupakis F, Intini R, Cremolini C, Orlandi A, Sartore-Bianchi A, Pietrantonio F, et al. A validated prognostic classifier for V600EBRAF-mutated metastatic colorectal cancer: the “BRAF BeCool” study. Eur J Cancer. 2019;118:121–30. https://doi.org/10.1016/j.ejca.2019.06.008
Caris Life Sciences. Caris Molecular Intelligence Tumor Profiling—Enabling Precision Medicine n.d. 2021. https://www.carismolecularintelligence.com/. Accessed Nov 2021.
Cremolini C, Schirripa M, Antoniotti C, Moretto R, Salvatore L, Masi G, et al. First-line chemotherapy for mCRC—a review and evidence-based algorithm. Nat Rev Clin Oncol. 2015;12:607–19. https://doi.org/10.1038/nrclinonc.2015.129
Loupakis F, Yang D, Yau L, Feng S, Cremolini C, Zhang W, et al. Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst. 2015;107:dju427. https://doi.org/10.1093/jnci/dju427
Salem ME, Puccini A, Grothey A, Raghavan D, Goldberg RM, Xiu J, et al. Landscape of tumor mutation load, mismatch repair deficiency, and PD-L1 expression in a large patient cohort of gastrointestinal cancers. Mol Cancer Res. 2018;16:805–12. https://doi.org/10.1158/1541-7786.MCR-17-0735
Vanderwalde A, Spetzler D, Xiao N, Gatalica Z, Marshall J. Microsatellite instability status determined by next-generation sequencing and compared with PD-L1 and tumor mutational burden in 11,348 patients. Cancer Med. 2018;7:746–56. https://doi.org/10.1002/cam4.1372
Abraham JP, Magee D, Cremolini C, Antoniotti C, Halbert DD, Xiu J, et al. Clinical validation of a machine-learning-derived signature predictive of outcomes from first-line oxaliplatin-based chemotherapy in advanced colorectal cancer. Clin Cancer Res. 2021;27:1174–83. https://doi.org/10.1158/1078-0432.CCR-20-3286
Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21:1353–65. https://doi.org/10.1016/S1470-2045(20)30445-9
Merino DM, McShane LM, Fabrizio D, Funari V, Chen S-J, White JR, et al. Establishing guidelines to harmonize tumor mutational burden (TMB): in silico assessment of variation in TMB quantification across diagnostic platforms: phase I of the Friends of Cancer Research TMB Harmonization Project. J Immunother Cancer. 2020;8:e000147. https://doi.org/10.1136/jitc-2019-000147
Becht E, Reyniès A, de, Giraldo NA, Pilati C, Buttard B, Lacroix L, et al. Immune and stromal classification of colorectal cancer is associated with molecular subtypes and relevant for precision immunotherapy. Clin Cancer Res. 2016;22:4057–66. https://doi.org/10.1158/1078-0432.CCR-15-2879
Barras D, Missiaglia E, Wirapati P, Sieber OM, Jorissen RN, Love C, et al. BRAF V600E mutant colorectal cancer subtypes based on gene expression. Clin Cancer Res. 2017;23:104–15. https://doi.org/10.1158/1078-0432.CCR-16-0140
Kleeman SO, Koelzer VH, Jones HJ, Vazquez EG, Davis H, East JE, et al. Exploiting differential Wnt target gene expression to generate a molecular biomarker for colorectal cancer stratification. Gut. 2020;69:1092–103. https://doi.org/10.1136/gutjnl-2019-319126
Moretto R, Elliott A, Zhang J, Arai H, Germani MM, Conca V, et al. Homologous recombination deficiency alterations in colorectal cancer: clinical, molecular, and prognostic implications. J Natl Cancer Inst. 2021. https://doi.org/10.1093/jnci/djab169.
Coleman RL, Swisher EM, Oza AM, Scott CL, Giordano H, Lin KK, et al. Refinement of prespecified cutoff for genomic loss of heterozygosity (LOH) in ARIEL2 part 1: a phase II study of rucaparib in patients (pts) with high grade ovarian carcinoma (HGOC). J Clin Oncol. 2016;34:5540–5540. https://doi.org/10.1200/JCO.2016.34.15_suppl.5540
Coleman RL, Brady MF, Herzog TJ, Sabbatini P, Armstrong DK, Walker JL, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:779–91. https://doi.org/10.1016/S1470-2045(17)30279-6
Souglakos J, Philips J, Wang R, Marwah S, Silver M, Tzardi M, et al. Prognostic and predictive value of common mutations for treatment response and survival in patients with metastatic colorectal cancer. Br J Cancer. 2009;101:465–72. https://doi.org/10.1038/sj.bjc.6605164
Clancy C, Burke JP, Kalady MF, Coffey JC. BRAF mutation is associated with distinct clinicopathological characteristics in colorectal cancer: a systematic review and meta-analysis. Colorectal Dis. 2013;15:e711–718. https://doi.org/10.1111/codi.12427
Venderbosch S, Nagtegaal ID, Maughan TS, Smith CG, Cheadle JP, Fisher D, et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res. 2014;20:5322–30. https://doi.org/10.1158/1078-0432.CCR-14-0332
Franko J, Shi Q, Meyers JP, Maughan TS, Adams RA, Seymour MT, et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016;17:1709–19. https://doi.org/10.1016/S1470-2045(16)30500-9
Loupakis F, Moretto R, Aprile G, Muntoni M, Cremolini C, Iacono D, et al. Clinico-pathological nomogram for predicting BRAF mutational status of metastatic colorectal cancer. Br J Cancer. 2016;114:30–6. https://doi.org/10.1038/bjc.2015.399
Kopetz S, Grothey A, Yaeger R, Van Cutsem E, Desai J, Yoshino T, et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E–mutated colorectal cancer. N Engl J Med. 2019;381:1632–43. https://doi.org/10.1056/NEJMoa1908075
Tabernero J, Grothey A, Van Cutsem E, Yaeger R, Wasan H, Yoshino T, et al. Encorafenib plus cetuximab as a new standard of care for previously treated BRAF V600E–mutant metastatic colorectal cancer: updated survival results and subgroup analyses from the BEACON study. J Clin Oncol. 2021;39:273–84. https://doi.org/10.1200/JCO.20.02088
Grothey A, Tabernero J, Taieb J, Yaeger R, Yoshino T, Maiello E, et al. LBA-5 ANCHOR CRC: a single-arm, phase 2 study of encorafenib, binimetinib plus cetuximab in previously untreated BRAF V600E-mutant metastatic colorectal cancer. Ann Oncol. 2020;31:S242–3. https://doi.org/10.1016/j.annonc.2020.04.080
Kopetz S, Grothey A, Yaeger R, Ciardiello F, Desai J, Kim TW, et al. BREAKWATER: Randomized phase 3 study of encorafenib (enco) + cetuximab (cet) ± chemotherapy for first-line treatment (tx) of BRAF V600E-mutant (BRAFV600) metastatic colorectal cancer (mCRC). J Clin Oncol. 2022;40:TPS211–TPS211. https://doi.org/10.1200/JCO.2022.40.4_suppl.TPS211
André T, Shiu K-K, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med. 2020;383:2207–18. https://doi.org/10.1056/NEJMoa2017699
Gelsomino F, Barbolini M, Spallanzani A, Pugliese G, Cascinu S. The evolving role of microsatellite instability in colorectal cancer: a review. Cancer Treat Rev. 2016;51:19–26. https://doi.org/10.1016/j.ctrv.2016.10.005
Sadanandam A, Lyssiotis CA, Homicsko K, Collisson EA, Gibb WJ, Wullschleger S, et al. A colorectal cancer classification system that associates cellular phenotype and responses to therapy. Nat Med. 2013;19:619–25. https://doi.org/10.1038/nm.3175
Song N, Pogue-Geile KL, Gavin PG, Yothers G, Kim SR, Johnson NL, et al. Clinical Outcome from oxaliplatin treatment in stage II/III colon cancer according to intrinsic subtypes: secondary analysis of NSABP C-07/NRG oncology randomized clinical trial. JAMA Oncol. 2016;2:1162–9. https://doi.org/10.1001/jamaoncol.2016.2314
Aderka D, Stintzing S, Heinemann V. Explaining the unexplainable: discrepancies in results from the CALGB/SWOG 80405 and FIRE-3 studies. Lancet Oncol. 2019;20:e274–83. https://doi.org/10.1016/S1470-2045(19)30172-X
Zaanan A, Bachet J-B, André T, Sinicrope FA. Prognostic impact of deficient DNA mismatch repair and mutations in KRAS, and BRAFV600E in patients with lymph node-positive colon cancer. Curr Colorectal Cancer Rep. 2014;10:346–53. https://doi.org/10.1007/s11888-014-0237-2
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467–80. https://doi.org/10.1016/S0140-6736(19)32319-0
Taieb J, Shi Q, Pederson L, Alberts S, Wolmark N, Van Cutsem E, et al. Prognosis of microsatellite instability and/or mismatch repair deficiency stage III colon cancer patients after disease recurrence following adjuvant treatment: results of an ACCENT pooled analysis of seven studies. Ann Oncol. 2019;30:1466–71. https://doi.org/10.1093/annonc/mdz208
Formica V, Sera F, Cremolini C, Riondino S, Morelli C, Arkenau H-T, et al. KRAS and BRAF mutations in stage II/III colon cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2021. https://doi.org/10.1093/jnci/djab190.
Weisenberger DJ, Siegmund KD, Campan M, Young J, Long TI, Faasse MA, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787–93. https://doi.org/10.1038/ng1834
Acknowledgements
We are grateful to the “BRAF BeCool” investigators from the participating Italian centres.
Funding
The present work was partially funded by Regione Veneto—grant RP-2014-00000395 and by Italian Health Ministry Grant 2019 GR-2019-12368903.
Author information
Authors and Affiliations
Contributions
Study concepts: RM, DR, CC, AE, WMK. Study design: RM, DR, CC, AE, WMK. Data acquisition: RM, DR, SL, PB, RI, VC, FP, AS-B, CA, CR, MS, MS, NP, MAC, MC, FC, GM. Quality control of the data and algorithms: RM, DR, PB. Data analysis and interpretation: RM, DR, CC, AE, PB. Statistical analysis: DR, AE, PB. Paper preparation: RM, DR, CC, AE. Paper editing: all authors. Paper review: all authors.
Corresponding author
Ethics declarations
Competing interests
AE: Employee of Caris Life Sciences. PB: Employee of Caris Life Sciences. FP: honoraria for speaker activities and participation in advisory boards from Sanofi, Amgen, Bayer, Merck-Serono, Lilly, Astrazeneca, Servier, Organon, MSD; and research grants from BMS and Astrazeneca. AS-B: Honoraria: Amgen, Bayer, MSD, Servier. Consulting or advisory role—Amgen, Bayer, Novartis, Sanofi, Servier. MS: Advisory board, speakers’ bureau MERCK, MSD, Sanofi, Amgen, BMS, EISAI, Servier. GM: Honraria—Amgen, F. HoffmaneLa Roche, Bayer, Merk Serono, Sirtex. SL: Speakers’ Bureau—Amgen, Merck, Roche, Lilly, Bristol-Myers Squibb, Pierre-Fabre, GSK and Servier. Consulting or advisory role—Amgen, MSD, Merck Serono, Lilly, Astra Zeneca, Incyte, Daiichi-Sankyo, Bristol-Myers Squibb, Servier, Research funding to Institution—Bayer, Merck, Amgen, Roche, Lilly, Astra Zeneca Bristol-Myers Squibb. WMK: Consultant—Merck. Employee of Caris Life Sciences. CC: honoraria—Amgen, Bayer, Merck, Roche and Servier. Consulting or advisory role—Amgen, Bayer, MSD, Roche. Speakers’ Bureau—Servier. Research funding—Bayer, Merck, Servier. Travel, accommodations and expenses—Roche and Servier. The remaining authors declare no competing interests.
Ethics approval and consent to participate
BRAF BeCool study was conducted in accordance with the Declaration of Helsinki. Approval for BRAF BeCool protocol was obtained from local ethics committees of participating sites.
Consent to publication
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Moretto, R., Elliott, A., Rossini, D. et al. Benefit from upfront FOLFOXIRI and bevacizumab in BRAFV600E-mutated metastatic colorectal cancer patients: does primary tumour location matter?. Br J Cancer 127, 957–967 (2022). https://doi.org/10.1038/s41416-022-01852-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41416-022-01852-0
This article is cited by
-
BRAFV600E Metastatic Colorectal Cancer: Perspective from a Patient, a Caregiver, and an Oncologist
Advances in Therapy (2023)