Abstract
Background
Micropapillary (MIP) component was a major concern in determining surgical strategy in lung adenocarcinoma (LUAD). We sought to develop a novel method for detecting MIP component during surgery.
Methods
Differentially expressed proteins between MIP-positive and MIP-negative LUAD were identified through proteomics analysis. The semi-dry dot-blot (SDB) method which visualises the targeted protein was developed to detect MIP component.
Results
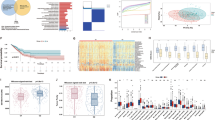
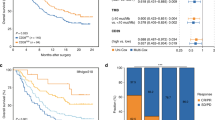
Cellular retinoic acid-binding protein 2 (CRABP2) was significantly upregulated in MIP-positive LUAD (P < 0.001), and the high CRABP2 expression zone showed spatial consistency with MIP component. CRABP2 expression was also associated with decreased recurrence-free survival (P < 0.001). In the prospective cohort, the accuracy and sensitivity of detecting MIP component using SDB method by visualising CRABP2 were 82.2% and 72.7%, which were comparable to these of pathologist. Pathologist with the aid of SDB method would improve greatly in diagnostic accuracy (86.4%) and sensitivity (78.2%). In patients with minor MIP component (≤5%), the sensitivity of SDB method (63.6%) was significantly higher than pathologist (45.4%).
Conclusions
Intraoperative examination of CRABP2 using SDB method to detect MIP component reached comparable performance to pathologist, and SDB method had notable superiority than pathologist in detecting minor MIP component.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Zhu E, Dai C, Xie H, Su H, Hu X, Li M, et al. Lepidic component identifies a subgroup of lung adenocarcinoma with a distinctive prognosis: a multicenter propensity-matched analysis. Ther Adv Med Oncol. 2020;12:1758835920982845.
Wang T, Deng J, She Y, Zhang L, Wang B, Ren Y, et al. Radiomics signature predicts the recurrence-free survival in stage I non-small cell lung cancer. Ann Thorac Surg. 2020;109:1741–9.
She Y, Jin Z, Wu J, Deng J, Zhang L, Su H, et al. Development and validation of a deep learning model for non-small cell lung cancer survival. JAMA Netw open. 2020;3:e205842.
Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–85.
Yanagawa N, Shiono S, Abiko M, Ogata SY, Sato T, Tamura G. New IASLC/ATS/ERS classification and invasive tumor size are predictive of disease recurrence in stage I lung adenocarcinoma. J Thorac Oncol. 2013;8:612–8.
Travis WD, Brambilla E, Van Schil P, Scagliotti GV, Huber RM, Sculier JP, et al. Paradigm shifts in lung cancer as defined in the new IASLC/ATS/ERS lung adenocarcinoma classification. Eur Respir J. 2011;38:239–43.
Warth A, Muley T, Meister M, Stenzinger A, Thomas M, Schirmacher P, et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol. 2012;30:1438–46. 7
Chen D, Ding Q, Wang W, Wang X, Wu X, Mao Y, et al. Characterization of extracapsular lymph node involvement and its clinicopathological characteristics in stage II-IIIA lung adenocarcinoma. Ann Surg Oncol. 2021;28:2088–98.
Yao J, Zhu E, Li M, Liu J, Zhang L, Ke H, et al. Prognostic impact of micropapillary component in patients with node-negative subcentimeter lung adenocarcinoma: a Chinese cohort study. Thorac Cancer. 2020;11:3566–75.
Pani E, Kennedy G, Zheng X, Ukert B, Jarrar D, Gaughan C, et al. Factors associated with nodal metastasis in 2-centimeter or less non-small cell lung cancer. J Thorac Cardiovasc Surg. 2020;159:1088–96.
Yuan Y, Ma G, Zhang Y, Chen H. Presence of micropapillary and solid patterns are associated with nodal upstaging and unfavorable prognosis among patient with cT1N0M0 lung adenocarcinoma: a large-scale analysis. J Cancer Res Clin Oncol. 2018;144:743–9.
Dai C, Xie H, Kadeer X, Su H, Xie D, Ren Y, et al. Relationship of lymph node micrometastasis and micropapillary component and their joint influence on prognosis of patients with stage I lung adenocarcinoma. Am J Surg Pathol. 2017;41:1212–20.
Nitadori J, Bograd AJ, Kadota K, Sima CS, Rizk NP, Morales EA, et al. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2 cm or smaller. J Natl Cancer Inst. 2013;105:1212–20.
Su H, Xie H, Dai C, Zhao S, Xie D, She Y, et al. Procedure-specific prognostic impact of micropapillary subtype may guide resection strategy in small-sized lung adenocarcinomas: a multicenter study. Ther Adv Med Oncol. 2020;12:1758835920937893.
Sun W, Su H, Liu J, Zhang L, Li M, Xie H, et al. Impact of histological components on selecting limited lymphadenectomy for lung adenocarcinoma ≤2 cm. Lung Cancer. 2020;150:36–43.
Xu Y, Ji W, Hou L, Lin S, Shi Y, Zhou C, et al. Enhanced CT-based radiomics to predict micropapillary pattern within lung invasive adenocarcinoma. Front Oncol. 2021;11:704994.
Huang KY, Ko PZ, Yao CW, Hsu CN, Fang HY, Tu CY, et al. Inaccuracy of lung adenocarcinoma subtyping using preoperative biopsy specimens. J Thorac Cardiovasc Surg. 2017;154:332–9.
Yeh YC, Nitadori J, Kadota K, Yoshizawa A, Rekhtman N, Moreira AL, et al. Using frozen section to identify histological patterns in stage I lung adenocarcinoma of < /=3 cm: accuracy and interobserver agreement. Histopathology. 2015;66:922–38.
Trejo Bittar HE, Incharoen P, Althouse AD, Dacic S. Accuracy of the IASLC/ATS/ERS histological subtyping of stage I lung adenocarcinoma on intraoperative frozen sections. Mod Pathol. 2015;28:1058–63.
Moreira AL, Ocampo PSS, Xia Y, Zhong H, Russell PA, Minami Y, et al. A grading system for invasive pulmonary adenocarcinoma: a proposal from the International Association for the Study of Lung Cancer Pathology Committee. J Thorac Oncol. 2020;15:1599–610.
Feng X, Zhang M, Wang B, Zhou C, Mu Y, Li J, et al. CRABP2 regulates invasion and metastasis of breast cancer through hippo pathway dependent on ER status. J Exp Clin cancer Res. 2019;38:361.
Zhao Y, Wang R, Shen X, Pan Y, Cheng C, Li Y, et al. Minor components of micropapillary and solid subtypes in lung adenocarcinoma are predictors of lymph node metastasis and poor prognosis. Ann Surg Oncol. 2016;23:2099–105.
Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60:615–22.
Kang HR, Cho JY, Lee SH, Lee YJ, Park JS, Cho YJ, et al. Role of low-dose computerized tomography in lung cancer screening among never-smokers. J Thorac Oncol. 2019;14:436–44.
Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409.
Wisnivesky JP, Henschke CI, Swanson S, Yankelevitz DF, Zulueta J, Marcus S, et al. Limited resection for the treatment of patients with stage IA lung cancer. Ann Surg. 2010;251:550–4.
Veluswamy RR, Ezer N, Mhango G, Goodman E, Bonomi M, Neugut AI, et al. Limited resection versus lobectomy for older patients with early-stage lung cancer: impact of histology. J Clin Oncol. 2015;33:3447–53.
Chiang XH, Lu TP, Hsieh MS, Tsai TM, Liao HC, Kao TN, et al. Thoracoscopic wedge resection versus segmentectomy for cT1N0 lung adenocarcinoma. Ann Surg Oncol. 2021;28:8398–411.
Tsutani Y, Miyata Y, Nakayama H, Okumura S, Adachi S, Yoshimura M, et al. Appropriate sublobar resection choice for ground glass opacity-dominant clinical stage IA lung adenocarcinoma: wedge resection or segmentectomy. Chest. 2014;145:66–71.
Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet. 2022;399:1607–17.
Altorki NK, Wang X, Wigle D, Gu L, Darling G, Ashrafi AS, et al. Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: post-hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503). Lancet Respir Med. 2018;6:915–24.
Caso R, Sanchez-Vega F, Tan KS, Mastrogiacomo B, Zhou J, Jones GD, et al. The underlying tumor genomics of predominant histologic subtypes in lung adenocarcinoma. J Thorac Oncol. 2020;15:1844–56.
Song SH, Park H, Lee G, Lee HY, Sohn I, Kim HS, et al. Imaging phenotyping using radiomics to predict micropapillary pattern within lung adenocarcinoma. J Thorac Oncol. 2017;12:624–32.
Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129:723–33.
Xie T, Tan M, Gao Y, Yang H. CRABP2 accelerates epithelial mesenchymal transition in serous ovarian cancer cells by promoting TRIM16 methylation via upregulating EZH2 expression. Environ Toxicol. 2022;37:1957–67.
Calmon MF, Rodrigues RV, Kaneto CM, Moura RP, Silva SD, Mota LD, et al. Epigenetic silencing of CRABP2 and MX1 in head and neck tumors. Neoplasia. 2009;11:1329–39.
Han SS, Kim WJ, Hong Y, Hong SH, Lee SJ, Ryu DR, et al. RNA sequencing identifies novel markers of non-small cell lung cancer. Lung Cancer. 2014;84:229–35.
Hirakawa H, Shibata K, Ohzono E. Use of a semi-dry dot-blot for rapid detection of lymph node metastasis. Clin Chim Acta. 2010;411:1149–50.
Hamada K, Otsubo R, Takeshita H, Nonaka T, Tominaga T, Hidaka S, et al. Diagnosis of lymph node metastasis in colorectal cancer by a semi-dry dot-blot method. Anticancer Res. 2019;39:2069–76.
Otsubo R, Hirakawa H, Oikawa M, Baba M, Inamasu E, Shibata K, et al. Validation of a novel diagnostic kit using the semidry dot-blot method to detect metastatic lymph nodes in breast cancer: distinguishing macrometastases from nonmacrometastases. Clin Breast Cancer. 2018;18:e345–e351.
Tomoshige K, Tsuchiya T, Otsubo R, Oikawa M, Yamasaki N, Matsumoto K, et al. Intraoperative diagnosis of lymph node metastasis in non-small-cell lung cancer by a semi-dry dot-blot method. Eur J Cardiothorac Surg. 2016;49:617–22.
Otsubo R, Oikawa M, Hirakawa H, Shibata K, Abe K, Hayashi T, et al. Novel diagnostic procedure for determining metastasis to sentinel lymph nodes in breast cancer using a semi-dry dot-blot method. Int J Cancer. 2014;134:905–12.
Fahrmann JF, Marsh T, Irajizad E, Patel N, Murage E, Vykoukal J, et al. Blood-based biomarker panel for personalized lung cancer risk assessment. J Clin Oncol. 2022;40:876–83.
Bigbee WL, Gopalakrishnan V, Weissfeld JL, Wilson DO, Dacic S, Lokshin AE, et al. A multiplexed serum biomarker immunoassay panel discriminates clinical lung cancer patients from high-risk individuals found to be cancer-free by CT screening. J Thorac Oncol. 2012;7:698–708.
Ostrin EJ, Bantis LE, Wilson DO, Patel N, Wang R, Kundnani D, et al. Contribution of a blood-based protein biomarker panel to the classification of indeterminate pulmonary nodules. J Thorac Oncol. 2021;16:228–36.
Guida F, Sun N, Bantis LE, Muller DC, Li P, Taguchi A, et al. Assessment of lung cancer risk on the basis of a biomarker panel of circulating proteins. JAMA Oncol. 2018;4:e182078.
Kim DJ, Kim WJ, Lim M, Hong Y, Lee SJ, Hong SH, et al. Plasma CRABP2 as a novel biomarker in patients with non-small cell lung cancer. J Korean Med Sci. 2018;33:e178.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Key Research and Development Program of China (grant no. 2022YFC2407401), the Shanghai Hospital Development Center (SHDC2020CR1021B and SHDC22021217), Shanghai Municipal Health Commission (202040322 and 20194Y0129), Shanghai Science and Technology Commission (20XD1403000 and 21S31905200).
Author information
Authors and Affiliations
Consortia
Contributions
Conception and design: LX, HS, SZ, DZ and CC; Administrative support: CW, DZ and CC; Provision of study materials or patients: CW, DZ and CC; Collection and assembly of data: HS, HX, YR, JG, FW, XX and CD. Data analysis and interpretation: LX, HS and SZ. Manuscript writing: All authors; Final approval of manuscript: All authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The institutional review board of Shanghai Pulmonary Hospital affiliated to Tongji University approved this study (IRB NO: L21-340).
Consent for publication
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, L., Su, H., Zhao, S. et al. Development of the semi-dry dot-blot method for intraoperative detecting micropapillary component in lung adenocarcinoma based on proteomics analysis. Br J Cancer 128, 2116–2125 (2023). https://doi.org/10.1038/s41416-023-02241-x
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41416-023-02241-x