Abstract
Background
Disorder of cell cycle represents as a major driver of hepatocarcinogenesis and constitutes an attractive therapeutic target. However, identifying key genes that respond to cell cycle-dependent treatments still facing critical challenges in hepatocellular carcinoma (HCC). Increasing evidence indicates that dynein light chain 1 (DYNLL1) is closely related to cell cycle progression and plays a critical role in tumorigenesis. In this study, we explored the role of DYNLL1 in the regulation of cell cycle progression in HCC.
Methods
We analysed clinical specimens to assess the expression and predictive value of DYNLL1 in HCC. The oncogenic role of DYNLL1 was determined by gain or loss-of-function experiments in vitro, and xenograft tumour, liver orthotopic, and DEN/CCl4-induced mouse models in vivo. Mass spectrometry analysis, RNA sequencing, co-immunoprecipitation assays, and forward and reverse experiments were performed to clarify the mechanism by which DYNLL1 activates the interleukin-2 enhancer-binding factor 2 (ILF2)/CDK4 signalling axis. Finally, the sensitivity of HCC cells to palbociclib and sorafenib was assessed by apoptosis, cell counting kit-8, and colony formation assays in vitro, and xenograft tumour models and liver orthotopic models in vivo.
Results
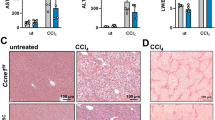
DYNLL1 was significantly higher in HCC tissues than that in normal liver tissues and closely related to the clinicopathological features and prognosis of patients with HCC. Importantly, DYNLL1 was identified as a novel hepatocarcinogenesis gene from both in vitro and in vivo evidence. Mechanistically, DYNLL1 could interact with ILF2 and facilitate the expression of ILF2, then ILF2 could interact with CDK4 mRNA and delay its degradation, which in turn activates downstream G1/S cell cycle target genes CDK4. Furthermore, palbociclib, a selective CDK4/6 inhibitor, represents as a promising therapeutic strategy for DYNLL1-overexpressed HCC, alone or particularly in combination with sorafenib.
Conclusions
Our work uncovers a novel function of DYNLL1 in orchestrating cell cycle to promote HCC development and suggests a potential synergy of CDK4/6 inhibitor and sorafenib for the treatment of HCC patients, especially those with increased DYNLL1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data needed to support the conclusions in the paper are present in the paper or the Supplementary Materials. Additional data related to this paper can be requested from the authors.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology. 2015;149:1226–39.
Xue W, Wang XW. The search for precision models clinically relevant to human liver cancer. Hepat Oncol. 2015;2:315–9.
Roberts AJ, Kon T, Knight PJ, Sutoh K, Burgess SA. Functions and mechanics of dynein motor proteins. Nat Rev Mol Cell Biol. 2013;14:713–26.
Singh PK, Weber A, Hacker G. The established and the predicted roles of dynein light chain in the regulation of mitochondrial apoptosis. Cell Cycle. 2018;17:1037–47.
Singh PK, Roukounakis A, Weber A, Das KK, Sohm B, Villunger A, et al. Dynein light chain binding determines complex formation and posttranslational stability of the Bcl-2 family members Bmf and Bim. Cell Death Differ. 2020;27:434–50.
Barbar E. Dynein light chain LC8 is a dimerization hub essential in diverse protein networks. Biochemistry. 2008;47:503–8.
King SM. Dynein-independent functions of DYNLL1/LC8: redox state sensing and transcriptional control. Sci Signal. 2008;1:e51.
Wong DM, Li L, Jurado S, King A, Bamford R, Wall M, et al. The transcription factor ASCIZ and its target DYNLL1 are essential for the development and expansion of MYC-driven B cell lymphoma. Cell Rep. 2016;14:1488–99.
Rayala SK, den Hollander P, Balasenthil S, Yang Z, Broaddus RR, Kumar R. Functional regulation of oestrogen receptor pathway by the dynein light chain 1. EMBO Rep. 2005;6:538–44.
He YJ, Meghani K, Caron MC, Yang C, Ronato DA, Bian J, et al. DYNLL1 binds to MRE11 to limit DNA end resection in BRCA1-deficient cells. Nature. 2018;563:522–6.
Becker JR, Cuella-Martin R, Barazas M, Liu R, Oliveira C, Oliver AW, et al. The ASCIZ-DYNLL1 axis promotes 53BP1-dependent non-homologous end joining and PARP inhibitor sensitivity. Nat Commun. 2018;9:5406.
Berkel C, Cacan E. DYNLL1 is hypomethylated and upregulated in a tumor stage- and grade-dependent manner and associated with increased mortality in hepatocellular carcinoma. Exp Mol Pathol. 2020;117:104567.
Zhang RY, Liu ZK, Wei D, Yong YL, Lin P, Li H, et al. UBE2S interacting with TRIM28 in the nucleus accelerates cell cycle by ubiquitination of p27 to promote hepatocellular carcinoma development. Signal Transduct Target Ther. 2021;6:64.
Ingham M, Schwartz GK. Cell-cycle therapeutics come of age. J Clin Oncol. 2017;35:2949–59.
Takahata S, Yu Y, Stillman DJ. The E2F functional analogue SBF recruits the Rpd3(L) HDAC, via Whi5 and Stb1, and the FACT chromatin reorganizer, to yeast G1 cyclin promoters. EMBO J. 2009;28:3378–89.
Masaki T, Shiratori Y, Rengifo W, Igarashi K, Yamagata M, Kurokohchi K, et al. Cyclins and cyclin-dependent kinases: comparative study of hepatocellular carcinoma versus cirrhosis. Hepatology. 2003;37:534–43.
Ohashi R, Gao C, Miyazaki M, Hamazaki K, Tsuji T, Inoue Y, et al. Enhanced expression of cyclin E and cyclin A in human hepatocellular carcinomas. Anticancer Res. 2001;21:657–62.
Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015;14:130–46.
Schettini F, De Santo I, Rea CG, De Placido P, Formisano L, Giuliano M, et al. CDK 4/6 inhibitors as single agent in advanced solid tumors. Front Oncol. 2018;8:608.
Llovet JM, Villanueva A, Lachenmayer A, Finn RS. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol. 2015;12:408–24.
Sheng J, Kohno S, Okada N, Okahashi N, Teranishi K, Matsuda F, et al. Treatment of retinoblastoma 1-intact hepatocellular carcinoma with cyclin-dependent kinase 4/6 inhibitor combination therapy. Hepatology. 2021;74:1971–93.
Fang Y, Zhan Y, Xie Y, Du S, Chen Y, Zeng Z, et al. Integration of glucose and cardiolipin anabolism confers radiation resistance of HCC. Hepatology. 2022;75:1386–401.
Zhu Y, Yang J, Xu D, Gao XM, Zhang Z, Hsu JL, et al. Disruption of tumor-associated macrophage trafficking by the osteopontin-induced colony-stimulating factor-1 signalling sensitises hepatocellular carcinoma to anti-PD-L1 blockade. Gut. 2019;68:1653–66.
Chen CY, Ezzeddine N, Shyu AB, Messenger RNA. half-life measurements in mammalian cells. Methods Enzymol. 2008;448:335–57.
Matthews HK, Bertoli C, de Bruin R. Cell cycle control in cancer. Nat Rev Mol Cell Biol. 2022;23:74–88.
Rapali P, Radnai L, Süveges D, Harmat V, Tölgyesi F, Wahlgren WY, et al. Directed evolution reveals the binding motif preference of the LC8/DYNLL hub protein and predicts large numbers of novel binders in the human proteome. PLoS One. 2011;6:e18818.
Rapali P, Szenes A, Radnai L, Bakos A, Pál G, Nyitray L. DYNLL/LC8: a light chain subunit of the dynein motor complex and beyond. FEBS J. 2011;278:2980–96.
Stallcup MR, Poulard C. Gene-specific actions of transcriptional coregulators facilitate physiological plasticity: evidence for a physiological coregulator code. Trends Biochem Sci. 2020;45:497–510.
Marchesini M, Ogoti Y, Fiorini E, Aktas Samur A, Nezi L, D’Anca M, et al. ILF2 is a regulator of RNA splicing and DNA damage response in 1q21-amplified multiple myeloma. Cancer Cell. 2017;32:88–100.
Guan D, Altan-Bonnet N, Parrott AM, Arrigo CJ, Li Q, Khaleduzzaman M, et al. Nuclear factor 45 (NF45) is a regulatory subunit of complexes with NF90/110 involved in mitotic control. Mol Cell Biol. 2008;28:4629–41.
Barber GN. The NFAR’s (nuclear factors associated with dsRNA): evolutionarily conserved members of the dsRNA binding protein family. RNA Biol. 2009;6:35–39.
Li Y, Wang M, Yang M, Xiao Y, Jian Y, Shi D, et al. Nicotine-Induced ILF2 facilitates nuclear mRNA export of pluripotency factors to promote stemness and chemoresistance in human esophageal cancer. Cancer Res. 2021;81:3525–38.
Zhang X, Bustos MA, Gross R, Ramos RI, Takeshima TL, Mills GB, et al. Interleukin enhancer-binding factor 2 promotes cell proliferation and DNA damage response in metastatic melanoma. Clin Transl Med. 2021;11:e608.
Zhang H, Che Y, Xuan B, Wu X, Li H. Serine hydroxymethyltransferase 2 (SHMT2) potentiates the aggressive process of oral squamous cell carcinoma by binding to interleukin enhancer-binding factor 2 (ILF2). Bioengineered. 2022;13:8785–97.
Bollard J, Miguela V, Ruiz de Galarreta M, Venkatesh A, Bian CB, Roberto MP, et al. Palbociclib (PD-0332991), a selective CDK4/6 inhibitor, restricts tumor growth in preclinical models of hepatocellular carcinoma. Gut. 2017;66:1286–96.
Berkel C, Cacan E. In silico analysis of DYNLL1 expression in ovarian cancer chemoresistance. Cell Biol Int. 2020;44:1598–605.
Du H, Le Y, Sun F, Li K, Xu Y. ILF2 directly binds and stabilizes CREB to stimulate malignant phenotypes of liver cancer cells. Anal Cell Pathol (Amst). 2019;2019:1575031.
Li D, Shen L, Zhang X, Chen Z, Huang P, Huang C, et al. LncRNA ELF3-AS1 inhibits gastric cancer by forming a negative feedback loop with SNAI2 and regulates ELF3 mRNA stability via interacting with ILF2/ILF3 complex. J Exp Clin Cancer Res. 2022;41:332.
Zhao M, Liu Y, Chang J, Qi J, Liu R, Hou Y, et al. ILF2 cooperates with E2F1 to maintain mitochondrial homeostasis and promote small cell lung cancer progression. Cancer Biol Med. 2019;16:771–83.
Chiu CL, Li CG, Verschueren E, Wen RM, Zhang D, Gordon CA, et al. NUSAP1 binds ILF2 to modulate R-loop accumulation and DNA damage in prostate cancer. Int J Mol Sci. 2023;24:6258.
Rigo F, Hua Y, Chun SJ, Prakash TP, Krainer AR, Bennett CF. Synthetic oligonucleotides recruit ILF2/3 to RNA transcripts to modulate splicing. Nat Chem Biol. 2012;8:555–61.
Wang C, Wang H, Lieftink C, du Chatinier A, Gao D, Jin G, et al. CDK12 inhibition mediates DNA damage and is synergistic with sorafenib treatment in hepatocellular carcinoma. Gut. 2020;69:727–36.
Shen S, Dean DC, Yu Z, Duan Z. Role of cyclin-dependent kinases (CDKs) in hepatocellular carcinoma: therapeutic potential of targeting the CDK signaling pathway. Hepatol Res. 2019;49:1097–108.
Acknowledgements
We thank all laboratory members of the Department of Pathology of Southern Medical University for their support and comments.
Funding
This study was supported by grants from the Guangdong Provincial Key Laboratory of Precision Medicine for Gastrointestinal Cancer (2020B121201004); The National Natural Science Foundation of China (81972631, 82073063, 82103595, 82273039); The Natural Science Foundation of Guangdong Province (2021A1515010989, 2022A1515010298, 2022A1515111142, 2023A1515010274, 2023A1515010980); Guangdong Provincial Regional Joint Fund-Youth Fund Project (2020A1515110006); The Foundation of President of Nanfang Hospital (2020B012, 2023B005); The China Postdoctoral Science Foundation (2023M741557); Outstanding Youth Development Scheme of Nanfang Hospital, Southern Medical University (2020J010); The Guangdong Provincial Major Talents Project (2019JC05Y361).
Author information
Authors and Affiliations
Contributions
This study was designed by Yuechen Liu, Zhenkang Li, Zhiyong Shen, and Haijun Deng. Yuechen Liu and Zhenkang Li carried out most of the experiments and drafted the manuscript. Jinchao Zhang, Wei Liu and Shenyuan Guan performed the animal breeding and made statistical analyses. Yizhi Zhan and Yuan Fang discussed the data and drew the diagrams. Haijun Deng, Zhiyong Shen and Yongsheng Li supervised the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All mice care and experiments were approved by the Institutional Animal Care and Use Committee of Nanfang Hospital, and all animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals”. The use of excised human specimens was approved by the Institute Research Medical Ethics Committee of Nanfang Hospital (Guangzhou, China).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Consent for publication All authors have provided their consent to publish the manuscript.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Li, Z., Zhang, J. et al. DYNLL1 accelerates cell cycle via ILF2/CDK4 axis to promote hepatocellular carcinoma development and palbociclib sensitivity. Br J Cancer 131, 243–257 (2024). https://doi.org/10.1038/s41416-024-02719-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41416-024-02719-2
This article is cited by
-
Integrated multi-omics analysis reveals NOL11 as a novel prognostic biomarker for hepatocellular carcinoma
BMC Cancer (2025)
-
The TEAD4-DYNLL1 axis accelerates cell cycle progression and augments malignant properties of lung adenocarcinoma cells
European Journal of Medical Research (2025)
-
Development of a MVI associated HCC prognostic model through single cell transcriptomic analysis and 101 machine learning algorithms
Scientific Reports (2025)
-
Matrix stiffness-related extracellular matrix signatures and the DYNLL1 protein promote hepatocellular carcinoma progression through the Wnt/β-catenin pathway
BMC Cancer (2024)
-
ILF2 protein is a promising serum biomarker for early detection of gastric cancer
BMC Cancer (2024)