Abstract
Background
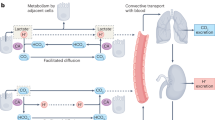
Tumor acidosis causes resistance to immune checkpoint blockade (ICB). We hypothesized that a “pH-sensitizer” can increase tumor extracellular pH (pHe) and improve tumor control following ICB. We also hypothesized that pHe measured with acidoCEST MRI can predict improved tumor control with ICB.
Methods
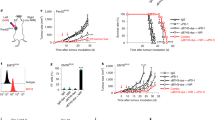
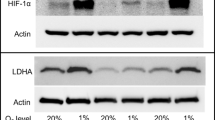
We tested the effects of pH-sensitizers on proton efflux rate (PER), cytotoxicity, T cell activation, tumor immunogenicity, tumor growth and survival using 4T1 and B16-F10 tumor cells. We measured in vivo tumor pHe of 4T1 and B16-F10 models with acidoCEST MRI.
Results
Among the pH-sensitizers tested, someprazole caused the greatest reduction in PER without exhibiting cytotoxicity or reducing T cell activation. Esomeprazole improved 4T1 tumor control with ICB administered one day after the pH-sensitizer. Tumor pHe positively correlated with TCF-1 + CD4 effector and CD8 T cell intratumoral frequencies and predicted improved 4T1 tumor control with ICB. For comparison, esomeprazole had a mild effect on B16-F10 tumor pHe, and worsened tumor control with ICB and increased intratumoral myeloid and dendritic cell (DC) frequencies.
Conclusions
A pH-sensitizer can improve tumor control with ICB, and acidoCEST MRI can be used to measure pHe and predict tumor control, but only in the 4T1 model and not the B16-F10 model.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data is available upon reasonable request to the corresponding author. The AcidoCEST code for Matlab 2022b is available on GitHub at https://github.com/CAMEL-MartyPagel/acidoCEST_MRI_Matlab.
References
Butterfield LH, Najjar YG. Immunotherapy combination approaches: mechanisms, biomarkers and clinical observations. Nature Rev Immunology 2024;24:399–416.
Lahiri A, Maji A, Potdar PD, Singh N, Parikh P, Bisht B, et al. Lung cancer immunotherapy: progress, pitfalls, and promises. Molec Cancer 2023;22:40.
Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 2015;161:205–14.
Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1480–92.
Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Costa EC, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N. Engl J Med. 2019;381:2020–31.
Motzer RJ, Tannir NM, McDermott DF, Frontera OA, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl J Med. 2018;378:1277–90.
Wong JSL, Kwok GGW, Tang V, Li BCW, Leung R, Chiu J, et al. Ipilimumab and nivolumab/pembrolizumab in advanced hepatocellular carcinoma refractory to prior immune checkpoint inhibitors. J Immunother Cancer. 2021;9:e001945.
Adams S, Othus M, Patel SP, Miller KD, Chugh R, Schuetze SM, et al. A multicenter phase II trial of ipilimumab and nivolumab in unresectable or metastatic metaplastic breast cancer: cohort 36 of dual anti-CTLA-4 and anti-PD-1 blockade in rare tumors (DART, SWOG S1609). Clin Cancer Res. 2022;28:271–8.
Warburg O. The metabolism of carcinoma cells. J Cancer Res. 1925;9:148–63.
Vaupel P, Multhoff G. Revisiting the Warburg effect: historical dogma versus current understanding. J Physiol. 2021;599:1745–57.
Gerweck LE, Seetharaman K. Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer Res. 1996;56:1194–8.
Gillies RJ, Raghunand N, Karczmar GS, Bhujwalla ZM. MRI of the tumor microenvironment. J Magn Reson Imaging. 2002;16:430–50.
Anemone A, Consolino L, Conti L, Irrera P, Hsu MY, Villano D, et al. Tumour acidosis evaluated in vivo by MRI-CEST pH imaging reveals breast cancer metastatic potential. Br J Cancer 2021;124:207–16.
Huntington KE, Louie A, Zhou L, Seyhan AA, Maxwell AW, El-Deiry WS. Colorectal cancer extracellular acidosis decreases immune cell killing and is partially ameliorated by pH-modulating agents that modify tumor cell cytokine profiles. Am J Cancer Res. 2022;12:138–51.
Davern M, Donlon NE, O’Connell F, Gaughan C, O’Donovan C, Habash M, et al. Acidosis significantly alters immune checkpoint expression profiles of T cells from oesophageal adenocarcinoma patients. Cancer Immunol Immunother. 2023;72:55–71.
Wei SC, Levine JH, Cogdill AP, Zhao Y, Anang NAAS, Andrews MC, et al. Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell 2017;170:1120–1133.e17.
Wei SC, Anang NAS, Sharma R, Andrews MC, Reuben A, Levine JH, et al. Combination anti-CTLA-4 plus anti-PD-1 checkpoint blockade utilizes cellular mechanisms partially distinct from monotherapies. Proc Natl Acad Sci USA. 2019;116:22699–709.
Calcinotto A, Filipazzi P, Grioni M, Iero M, De Milito A, Ricupito A, et al. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res. 2012;72:2746–56.
Knight FC, Gilchuk P, Kumar A, Becker KW, Sevimli S, Jacobson ME, et al. Mucosal immunization with a pH-responsive nanoparticle vaccine induces protective CD8+ lung-resident memory T cells. ACS Nano 2019;13:10939–60.
Ibrahim-Hashim A, Estrella V. Acidosis and cancer: from mechanism to neutralization. Cancer Metastasis Rev. 2019;38:149–55.
Gillies RJ, Ibrahim-Hashim A, Ordway B, Gatenby RA. Back to basic: trials and tribulations of alkalizing agents in cancer. Front Oncol. 2022;12:981718.
Shin S, Lee JY, Han J, Li FY, Ling DS, Park W. Tumor microenvironment modulating functional nanoparticles for effective cancer treatments. Tissue Eng. Regen Med. 2022;19:205–19.
de Maar JS, Sofias AM, Siegel TP, Vreeken RJ, Moonen C, Bos C, et al. Spatial heterogeneity of nanomedicine investigated by multiscale imaging of the drug, the nanoparticle and the tumour microenvironment. Theranostics 2020;10:1884–909.
Bailey KM, Wojtkowiak JW, Hashim AI, Gillies RJ. Targeting the metabolic microenvironment of tumors. Adv Pharmacol. 2012;65:63–107.
Feng Y, Xiong Y, Qiao T, Li X, Jia L, Han Y. Lactate dehydrogenase a: a key player in carcinogenesis and potential target in cancer therapy. Cancer Med. 2018;7:6124–36.
Pastorekova S, Gillies RJ. The role of carbonic anhydrase IX in cancer development: links to hypoxia, acidosis, and beyond. Cancer Metastasis Rev. 2019;38:65–77.
Bailey KM, Wojtkowiak JW, Cornnell HH, Ribeiro MC, Balagurunathan Y, Hashim AI, et al. Mechanisms of buffer therapy resistance. Neoplasia 2014;16:354–64.e1-3.
McDonald PC, Chia S, Bedard PL, Chu Q, Lyle M, Tang L, et al. A phase 1 study of SLC-0111, a novel inhibitor of carbonic anhydrase IX, in patients with advanced solid tumors. Am J Clin Oncol. 2020;43:484–90.
Nehme R, Hallal R, El Dor M, Kobeissy F, Gouilleux F, Mazurier F, et al. Repurposing of acriflavine to target chronic myeloid leukemia treatment. Curr Med. Chem. 2021;28:2218–33.
Scarpignato C, Gatta L, Zullo A, Blandizzi C. Effective and safe proton pump inhibitor therapy in acid-related diseases - A position paper addressing benefits and potential harms of acid suppression. BMC Medicine 2016;14:179.
Halford S, Veal GJ, Wedge SR, Payne GS, Bacon CM, Sloan P, et al. A phase I dose-escalation study of AZD3965, an oral monocarboxylate transporter 1 inhibitor, in patients with advanced cancer. Clin Cancer Res. 2023;29:1429–39.
Kulterer OC, Pfaff S, Wadsak W, Garstka N, Remzi M, Vraka C, et al. A microdosing study with (99m)Tc-PHC-102 for the SPECT/CT imaging of primary and metastatic lesions in renal cell carcinoma patients. J Nucl Med. 2021;62:360–5.
Brand K, Aichinger S, Forster S, Kupper S, Neumann B, Nurnberg W, et al. Cell-cycle-related metabolic and enzymatic events in proliferating rat thymocytes. Eur J Biochem. 1988;172:695–702.
Chen LQ, Randtke EA, Jones KM, Moon BF, Howison CM, Pagel MD. Evaluations of tumor acidosis within in vivo tumor models using parametric maps generated with acidoCEST MRI. Mol Imaging Biol. 2015;17:488–96.
Moon BF, Jones KM, Chen LQ, Liu P, Randke EA, Howison CM, et al. A comparison of iopromide and iopamidol, two acidoCEST MRI contrast media that measure tumor extracellular pH. Contrast Media Mol Imaging 2015;10:446–55.
Chen LQ, Howison CM, Jeffery JJ, Robey IF, Kuo PH, Pagel MD. Evaluations of extracellular pH within in vivo tumors using acidoCEST MRI. Magn Reson Med. 2014;72:1408–17.
Rupp T, Genest L, Babin D, Legrand C, Hunault M, Froget G, et al. Anti-CTLA-4 and anti-PD-1 immunotherapies repress tumor progression in preclinical breast and colon model with independent regulatory T cells response. Transl Oncol. 2022;20:101405.
Pilon-Thomas S, Kodumudi KN, El-Kenawi AE, Russell S, Weber AM, Luddy K, et al. Neutralization of tumor acidity improves antitumor responses to immunotherapy. Cancer Res. 2016;76:1381–90.
Anemone A, Consolino L, Arena F, Capozza M, Longo DL. Imaging tumor acidosis: a survey of the available techniques for mapping in vivo tumor pH. Cancer Metastasis Rev. 2019;38:25–49.
Kim K, Skora AD, Li Z, Liu Q, Tam AJ, Blosser RL, et al. Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proc Natl Acad Sci USA. 2014;111:11774–9.
Nakagawa Y, Negishi Y, Shimizu M, Takahashi M, Ichikawa M, Takahashi H. Effects of extracellular pH and hypoxia on the function and development of antigen-specific cytotoxic T lymphocytes. Immunol Lett. 2015;167:72–86.
Dowling CM, Hollinshead KER, Di Grande A, Pritchard J, Zhang H, Dillon ET, et al. Multiple screening approaches reveal HDAC6 as a novel regulator of glycolytic metabolism in triple-negative breast cancer. Sci Adv. 2021;7:eabc4897.
Skrzydlewski P, Twaruzek M, Grajewski J. Cytotoxicity of mycotoxins and their combinations on different cell lines: a review. Toxins 2022;14:244.
De Milito A, Canese R, Marino ML, Borghi M, Iero M, Villa A, et al. pH-dependent antitumor activity of proton pump inhibitors against human melanoma is mediated by inhibition of tumor acidity. Int J Cancer. 2010;127:207–19.
Irrera P, Roberto M, Consolino L, Anemone A, Villano D, Navarro-Tableros V, et al. Effect of esomeprazole treatment on extracellular tumor pH in a preclinical model of prostate cancer by MRI-CEST tumor pH imaging. Metabolites 2023;13:48.
Lindner K, Borchardt C, Schöpp M, Bürgers A, Stock C, Hussey DJ, et al. Proton pump inhibitors (PPIs) impact on tumour cell survival, metastatic potential and chemotherapy resistance, and affect expression of resistance-relevant miRNAs in esophageal cancer. J Exp Clin Cancer Res. 2014;33:73.
Rong A, Yao Y, Guo X, Jiang W, Jiang M, Yang J, et al. Precise cancer anti-acid therapy monitoring using pH-densitive MnO(2)@BSA nanoparticles by magnetic resonance imaging. ACS Appl Mater Interfaces 2021;13:18604–18.
Estrella V, Chen T, Lloyd M, Wojtkowiak J, Cornnell HH, Ibrahim-Hashim A, et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2013;73:1524–35.
Lechner MG, Karimi SS, Barry-Holson K, Angell TE, Murphy KA, Church CH, et al. Immunogenicity of murine solid tumor models as a defining feature of in vivo behavior and response to immunotherapy. J Immunother. 2013;36:477–89.
Bohn T, Rapp S, Luther N, Klein M, Bruehl TJ, Kojima N, et al. Tumor immunoevasion via acidosis-dependent induction of regulatory tumor-associated macrophages. Nat Immunol. 2018;19:1319–29.
Miller BC, Sen DR, Al Abosy R, Bi K, Virkud YV, LaFleur MW, et al. Subsets of exhausted CD8(+) T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol. 2019;20:326–36.
Chen Z, Ji Z, Ngiow SF, Manne S, Cai Z, Huang AC, et al. TCF-1-centered transcriptional network drives an effector versus exhausted CD8 T cell-fate decision. Immunity. 2019;51:840–855.e5.
Fan X, Quezada SA, Sepulveda MA, Sharma P, Allison JP. Engagement of the ICOS pathway markedly enhances efficacy of CTLA-4 blockade in cancer immunotherapy. J Exp Med. 2014;211:715–25.
Ricca JM, Oseledchyk A, Walther T, Liu C, Mangarin L, Merghoub T, et al. Pre-existing immunity to oncolytic virus potentiates its immunotherapeutic efficacy. Mol Ther. 2018;26:1008–19.
Knopf P, Stowbur D, Hoffmann SHL, Hermann N, Maurer A, Bucher V, et al. Acidosis-mediated increase in IFN-γ-induced PD-L1 expression on cancer cells as an immune escape mechanism in solid tumors. Mol Cancer. 2023;22:207.
Kwon YJ, Seo EB, Jeong AJ, Lee SH, Noh KH, Lee S, et al. The acidic tumor microenvironment enhances PD-L1 expression via activation of STAT3 in MDA-MB-231 breast cancer cells. BMC Cancer. 2022;22:852.
Rahman A, Janic B, Rahman T, Singh H, Ali H, Rattan R, et al. Immunotherapy enhancement by targeting extracellular tumor pH in triple-negative breast cancer mouse model. Cancers. 2023;15:4931.
Huntington KE, Louie A, Zhou LL, Seyhan AA, Maxwell AWP, El-Deiry WS. Colorectal cancer extracellular acidosis decreases immune cell killing and is partially ameliorated by pH-modulating agents that modify tumor cell cytokine profiles. Am J Cancer Res. 2022;12:138–51.
Giatromanolaki A, Koukourakis IM, Balaska K, Mitrakas AG, Harris AL, Koukourakis MI. Programmed death-1 receptor (PD-1) and PD-ligand-1 (PD-L1) expression in non-small cell lung cancer and the immune-suppressive effect of anaerobic glycolysis. Med. Oncol. 2019;36:76.
Acknowledgements
The authors thank the MD Anderson Cancer Center Small Animal Imaging Facility and the MD Anderson Cancer Center Advanced Cytometry & Sorting Facility for use of their resources.
Funding
This work was supported by a CPRIT grant (RP220270).
Author information
Authors and Affiliations
Contributions
RLT, SS, and MDP developed the experimental design. RLT and SS performed Seahorse studies. RLT and FWS developed the in vitro cell cultures and tumor models. RLT performed tumor growth and immunogeneicity studies. RLT and JDLC performed acidoCEST MRI studies. RLT, TL, and ASK performed image analyses. RLT, SP, PKB, SS, and MDP interpreted the results. RLC and MDP wrote the manuscript and all co-authors approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All experiments involving mice were conducted according to Protocol 00001998 approved by the Institutional Animal Care and Use Committee at the UT MD Anderson Cancer Center. All mice were housed in a pathogen-free facility fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tran, R.L., Li, T., de la Cerda, J. et al. Potentiation of immune checkpoint blockade with a pH-sensitizer as monitored in two pre-clinical tumor models with acidoCEST MRI. Br J Cancer 132, 744–753 (2025). https://doi.org/10.1038/s41416-025-02962-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41416-025-02962-1