Abstract
Background
Adjuvant BRAF/MEK inhibitors (BRAF/MEK) and anti-PD-1 therapy have become the standard care in resected stage III/IV melanoma. A head-to-head clinical trial comparison is lacking.
Methods
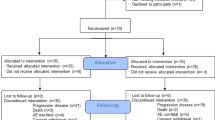
All stage III BRAF-mutant melanoma patients who received adjuvant BRAF/MEK or anti-PD-1 (2018-2022) were included from the Dutch Melanoma Treatment Registry. Propensity score matching (PSM) was used to compare 1- and 2-year recurrence-free survival (RFS), distant metastasis-free survival (DMFS), and overall survival (OS), and toxicity rates.
Findings
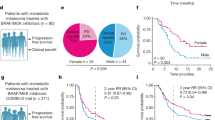
Among 952 patients (226 BRAF/MEK and 726 anti-PD-1), BRAF/MEK-treated patients had lower disease stages (16·4% versus 9·9% stage IIIA; p < 0·01) and more often comorbidities (75·2% versus 63·4%; p < 0·01). Median follow-up was 29 months. PSM created two similar groups of 223 patients. RFS, DMFS, and OS were not significantly different before and after PSM. Two-year RFS was 62% (95% CI 55–71) for BRAF/MEK and 65% (95% CI 58–72) for anti-PD-1; 2-year DMFS was 81% (95% CI 74–87) versus 81% (95% CI 75–86); 2-year OS was 86% (95% CI 81–92) versus 89% (95% CI 85–94), respectively. Grade ≥ 3 toxicity was reported in 11·7% (BRAF/MEK) and 13·4% (anti-PD-1).
Conclusion
After PSM, no significant differences in outcomes were observed between adjuvant anti-PD-1 and BRAF/MEK-treated patients with stage III BRAF-mutant melanoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Research data is available upon reasonable request.
References
Long GV, Hauschild A, Santinami M, Atkinson V, Mandalà M, Chiarion-Sileni V, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377:1813–23.
Long GV, Hauschild A, Santinami M, Kirkwood JM, Atkinson V, Mandala M, et al. Final results for adjuvant dabrafenib plus trametinib in stage III melanoma. N Engl J Med. 2024;391:1709–20.
Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377:1824–35.
Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N. Engl J Med. 2018;378:1789–801.
Eggermont AMM, Kicinski M, Blank CU, Mandala M, Long G V., Atkinson V, et al. Five-year analysis of adjuvant pembrolizumab or placebo in stage III melanoma. NEJM Evidence. 2022;1:EVIDoa2200214.
Larkin J, Del Vecchio Vecchio M, Mandalá M, Gogas H, Arance Fernandez Fernandez AM, Dalle S, et al. Adjuvant nivolumab versus ipilimumab in resected stage III/IV melanoma: 5-year efficacy and biomarker results from checkmate 238. Clin Cancer Res. 2023;29:3352–61.
De Meza MM, Blokx WAM, Bonenkamp JJ, Blank CU, Aarts MJB, van den Berkmortel FWPJ, et al. Adjuvant BRAF-MEK inhibitors versus anti PD-1 therapy in stage III melanoma: a propensity-matched outcome analysis. Cancers. 2023;15:409.
Bai X, Shaheen A, Grieco C, d’Arienzo PD, Mina F, Czapla JA, et al. Dabrafenib plus trametinib versus anti-PD-1 monotherapy as adjuvant therapy in BRAF V600-mutant stage III melanoma after definitive surgery: a multicenter, retrospective cohort study. EClinicalMedicine. 2023;65:102290.
Lodde GC, Hassel J, Wulfken LM, Meier F, Mohr P, Kähler K, et al. Adjuvant treatment and outcome of stage III melanoma patients: results of a multicenter real-world German Dermatologic Cooperative Oncology Group (DeCOG) study. Eur J Cancer. 2023;191:112957.
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46:399–424.
Austin PC. The use of propensity score methods with survival or time-to-event outcomes: Reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33:1242–58.
Jochems A, Schouwenburg MG, Leeneman B, Franken MG, Van Den Eertwegh AJM, Haanen JBAG, et al. Dutch melanoma treatment registry: quality assurance in the care of patients with metastatic melanoma in the Netherlands. Eur J Cancer. 2017;72:156–65.
van Zeijl MCT, de Wreede LC, van den Eertwegh AJM, Wouters MWJM, Jochems A, Schouwenburg MG, et al. Survival outcomes of patients with advanced melanoma from 2013 to 2017: Results of a nationwide population-based registry. Eur J Cancer. 2021;144:242–51.
Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, Long GV, Ross MI, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer (AJCC) eighth edition cancer staging manual. CA Cancer J Clin 2017;67:472–92.
Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–6.
Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–61.
Haist M, Stege H, Rogall F, Tan Y, Von Wasielewski I, Klespe KC, et al. Treatment management for BRAF-mutant melanoma patients with tumor recurrence on adjuvant therapy: a multicenter study from the prospective skin cancer registry ADOREG. J Immunother Cancer. 2023;11:e007630.
De Falco V, Suarato G, Napolitano R, Argenziano G, Famiglietti V, Amato A, et al. Real-world clinical outcome and safety of adjuvant therapy in stage III melanoma patients: data from two Academic Italian Institutions. Int J Cancer. 2023;153:133–40.
Schumann K, Mauch C, Klespe KC, Loquai C, Nikfarjam U, Schlaak M, et al. Real-world outcomes using PD-1 antibodies and BRAF + MEK inhibitors for adjuvant melanoma treatment from 39 skin cancer centers in Germany, Austria and Switzerland. J Eur Acad Dermatol Venereol. 2023;37:894–906.
Livingstone E, Forschner A, Hassel JC, von Wasielewski I, Meier F, Mohr P, et al. 1124P Adjuvant treatment of patients with stage III melanoma: 4-year follow-up time of multicenter real-world study. Ann Oncol. 2024;35:S740.
Dummer R, Hauschild A, Santinami M, Atkinson V, Mandalà M, Kirkwood JM, et al. Five-year analysis of adjuvant dabrafenib plus trametinib in stage III melanoma. N. Engl J Med. 2020;383:1139–48.
Eggermont AMM, Blank CU, Mandalà M, Long GV, Atkinson VG, Dalle S, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22:643–54.
de Meza MM, Ismail RK, Rauwerdink D, van Not OJ, van Breeschoten J, Blokx WAM, et al. Adjuvant treatment for melanoma in clinical practice—Trial versus reality. Eur J Cancer. 2021;158:234–45.
Suijkerbuijk KPM, van Eijs MJM, van Wijk F, Eggermont AMM. Clinical and translational attributes of immune-related adverse events. Nat Cancer. 2024;5:557–71.
Funding
The author(s) received no specific funding for this work. This study was supported by the Dutch Melanoma Treatment Registry (DMTR); the Dutch Institute for Clinical Auditing foundation received a start-up grant from governmental organization the Netherlands Organization for Health Research and Development (ZonMW, project number 836002002). The DMTR is structurally funded by Bristol Myers Squibb, Merck Sharpe & Dohme, Novartis, and Roche Pharma. Roche Pharma stopped funding in 2019, Novartis stopped funding in 2023, and Pierre Fabre started funding the DMTR in 2019.
Author information
Authors and Affiliations
Contributions
Bloem: Conceptualization, data curation, formal analysis, methodology, investigation, project administration, writing—original draft, and writing—review & editing. De Meza: Conceptualization, methodology, project administration, writing—original draft, and writing—review & editing. van den Eertwegh: Conceptualization, investigation, methodology, project administration, supervision, writing—review & editing. Aarts: Investigation, project administration, writing—review & editing. van den Berkmortel: Investigation, project administration, writing—review & editing. Blank: Investigation, project administration, writing—review & editing. Blokx: Investigation, project administration, writing—review & editing. Boers-Sonderen: Investigation, project administration, writing—review & editing. Bonenkamp: Investigation, project administration, writing—review & editing. Boreel: Investigation, project administration, writing—review & editing. de Groot: Investigation, project administration, writing—review & editing. Haanen: Investigation, project administration, writing—review & editing. Hospers: Investigation, project administration, writing—review & editing. Kapiteijn: Investigation, project administration, writing—review & editing. van Not.: Investigation, project administration, writing—review & editing. Piersma: Investigation, project administration, writing—review & editing. Rikhof: Investigation, project administration, writing—review & editing. Stevense-den Boer: Investigation, project administration, writing—review & editing. van der Veldt: Investigation, project administration, writing—review & editing. Vreugdenhil: Investigation, project administration, writing—review & editing. Suijkerbuijk: Conceptualization, investigation, methodology, project administration, supervision, writing—review & editing. Wouters: Conceptualization, investigation, methodology, project administration, supervision, writing—review & editing.
Corresponding author
Ethics declarations
Competing interests
AE participates in the Advisory Boards of Bristol-Myers Squibb, MSD Oncology, Ipsen, Pierre Fabre, Janssen Cilag BV. All fees were paid to the institution. GH has received grants from Seerave and Bristol-Myers Squibb, paid to the institution. GH participates in the Advisory Boards of Bristol-Myers Squibb, Roche, MSD, Novartis, Sanofi, Pierre Fabre, and Amgen. All fees were paid to the institution. KS has received grants from AbbVie, Novartis, Genmab, Philips, and Pierre Fabre, and participates in the Advisory Boards of AbbVie and Sairopa. All fees were paid to the institution. MA has received personal payment for the GUCS review; a national, online presentation “Highlight medical oncologie GU” in the Netherlands. MA received personal support from ASCO (2022) to attend the meeting. MA participated in the Advisory Boards of Amgen, Bristol Myers Squibb, Novartis, MSD-Merck, Merck-Pfizer, Pierre Fabre, Sanofi, Astellas, Bayer, and received a research grant from Merck-Pfizer. All fees were paid personally. AV participates in the Advisory Boards of Bristol-Myers Squibb, MSD, Ipsen, Eisai, Roche, Novartus, Sanofi, and Pfizer. All fees were paid to the institution. DP has received payment from Novartis for education about melanoma for new employees and from BMS for case writing for educational purposes. DP participates in the Advisory Boards of Peirre Fabre.
Ethics approval
The medical ethical committee considered the DMTR not subject to the Medical Research Involving Human Subjects Act under Dutch regulations since all data is anonymized, eliminating the need for informed consent. The study was performed in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bloem, M., de Meza, M.M., van den Eertwegh, A.J.M. et al. Adjuvant BRAF/MEK versus anti-PD-1 in BRAF-mutant melanoma: a propensity score matched survival analysis. Br J Cancer 132, 1124–1130 (2025). https://doi.org/10.1038/s41416-025-03021-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41416-025-03021-5