Abstract
Background
Ipilimumab+nivolumab (IPINIVO) can induce durable responses in advanced melanoma, but many patients experience progression at some point. It is currently unknown to what extent these patients benefit from IPINIVO rechallenge. This study describes efficacy and safety of IPINIVO rechallenge.
Methods
Data from advanced melanoma patients rechallenged with IPINIVO after previous ipilimumab-containing treatment were retrieved from the nationwide Dutch Melanoma Treatment Registry. Patient characteristics, responses, survival, and safety were analyzed.
Results
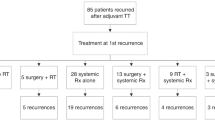
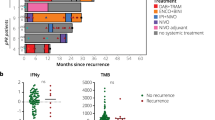
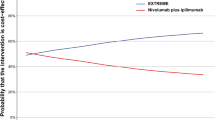
Among 3.759 patients receiving ipilimumab-containing treatment, 73 received rechallenge IPINIVO. 41 received IPINIVO, 32 ipilimumab monotherapy (IPI) as initial therapy. Objective response to rechallenge IPINIVO was seen in 36.1% (initial IPINIVO) and 40.0% (initial IPI) of patients. Median progression-free survival after rechallenge was 2.8 months (initial IPINIVO) and 5.6 months (initial IPI), but reached 18.4 months for responders to rechallenge therapy. Grade ≥3 immune-related adverse events occurred in 40.5% (initial IPINIVO) and 38.7% (initial IPI) of patients. Objective responses to initial and rechallenge treatment were discordant in 48.6% (initial IPINIVO) and 53.3% (initial IPI) of patients. Fifteen patients (20.5%) responded to rechallenge therapy but not to initial treatment.
Conclusions
Rechallenge IPINIVO after previous ipilimumab-based therapy had a considerable response rate, acceptable safety profile, and potential for a durable response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during this study are not publicly available due to privacy regulations in the Netherlands. Requests for data sharing can be addressed to the corresponding author.
References
Wolchok JD, Chiarion-Sileni V, Rutkowski P, Cowey CL, Schadendorf D, Wagstaff J, et al. Final, 10-year outcomes with nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2025;392:11–22.
Atkins MB, Lee SJ, Chmielowski B, Tarhini AA, Cohen GI, Truong TG, et al. Combination dabrafenib and trametinib versus combination nivolumab and ipilimumab for patients with advanced BRAF-mutant melanoma: the DREAMseq trial-ECOG-ACRIN EA6134. J Clin Oncol. 2022;41:186–97.
Ascierto PA, Mandaì M, Ferrucci PF, Guidoboni M, Rutkowski P, Ferraresi V, et al. Sequencing of ipilimumab plus nivolumab and encorafenib plus binimetinib for untreated BRAF-mutated metastatic melanoma (SECOMBIT): a randomized, three-arm, open-label phase II trial. J Clin Oncol. 2022;41:212–21.
Schreuer M, Jansen Y, Planken S, Chevolet I, Seremet T, Kruse V, et al. Combination of dabrafenib plus trametinib for BRAF and MEK inhibitor pretreated patients with advanced BRAFV600-mutant melanoma: an open-label, single arm, dual-centre, phase 2 clinical trial. Lancet Oncol. 2017;18:464–72.
Cremolini C, Rossini D, Dell’Aquila E, Lonardi S, Conca E, Del Re M, et al. Rechallenge for patients with RAS and BRAF wild-type metastatic colorectal cancer with acquired resistance to first-line cetuximab and irinotecan: a phase 2 single-arm clinical trial. JAMA Oncol. 2019;5:343–50.
Kang YK, Ryu MH, Yoo C, Ryoo BY, Kim HJ, Lee JJ, et al. Resumption of imatinib to control metastatic or unresectable gastrointestinal stromal tumours after failure of imatinib and sunitinib (RIGHT): A randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2013;14:1175–82.
Van Not OJ, van den Eertwegh AJM, Haanen JB, van Rijn RS, Aarts MJB, van den Berkmortel FWPJ, et al. BRAF/MEK inhibitor rechallenge in advanced melanoma patients. Cancer. 2024;130:1673–83.
Dolladille C, Ederhy S, Sassier M, Cautela J, Thuny F, Cohen AA, et al. Immune Checkpoint Inhibitor Rechallenge after Immune-Related Adverse Events in Patients with Cancer. JAMA Oncol. 2020;6:865–71.
Zhao Q, Zhang J, Xu L, Yang H, Liang N, Zhang L, et al. Safety and efficacy of the rechallenge of immune checkpoint inhibitors after immune-related adverse events in patients with cancer: a systemic review and meta-analysis. Vol. 12, Frontiers in Immunology. Frontiers Media S.A.; 2021. p. 730320.
Pires da Silva I, Ahmed T, Reijers ILM, Weppler AM, Betof Warner A, Patrinely JR, et al. Ipilimumab alone or ipilimumab plus anti-PD-1 therapy in patients with metastatic melanoma resistant to anti-PD-(L)1 monotherapy: a multicentre, retrospective, cohort study. Lancet Oncol. 2021;22:836–47.
VanderWalde A, Bellasea SL, Kendra KL, Khushalani NI, Campbell KM, Scumpia PO, et al. Ipilimumab with or without nivolumab in PD-1 or PD-L1 blockade refractory metastatic melanoma: a randomized phase 2 trial. Nat Med. 2023;29:2278–85.
Hepner A, Atkinson VG, Larkin J, Burrell RA, Carlino MS, Johnson DB, et al. Re-induction ipilimumab following acquired resistance to combination ipilimumab and anti–PD-1 therapy. Eur J Cancer. 2021;153:213–22.
Jochems A, Schouwenburg MG, Leeneman B, Franken MG, Van Den Eertwegh AJM, Haanen JBAG, et al. Dutch melanoma treatment registry: quality assurance in the care of patients with metastatic melanoma in the Netherlands. Eur J Cancer. 2017;72:156–65.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Asher N, Israeli-Weller N, Shapira-Frommer R, Ben-Betzalel G, Schachter J, Meirson T, et al. Immunotherapy discontinuation in metastatic melanoma: Lessons from real-life clinical experience. Cancers (Basel). 2021;13:3074.
Blasig H, Bender C, Hassel JC, Eigentler TK, Sachse MM, Hiernickel J, et al. Reinduction of PD1-inhibitor therapy: first experience in eight patients with metastatic melanoma. Melanoma Res. 2017;27:321–5.
Jansen YJL, Rozeman EA, Mason R, Goldinger SM, Geukes Foppen MH, Hoejberg L, et al. Discontinuation of anti-PD-1 antibody therapy in the absence of disease progression or treatment limiting toxicity: clinical outcomes in advanced melanoma. Ann Oncol. 2019;30:1154–61.
Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol. 2019;30:582–8.
Robert C, Ribas A, Schachter J, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019;20:1239–51.
Nardin C, Hennemann A, Diallo K, Funck-Brentano E, Puzenat E, Heidelberger V, et al. Efficacy of immune checkpoint inhibitor (ICI) rechallenge in advanced melanoma patients’ responders to a first course of ICI: a multicenter national retrospective study of the french group of skin cancers (Groupe de Cancérologie Cutanée, GCC). Cancers. 2023;15:3564.
Nomura M, Otsuka A, Kondo T, Nagai H, Nonomura Y, Kaku Y, et al. Efficacy and safety of retreatment with nivolumab in metastatic melanoma patients previously treated with nivolumab. Cancer Chemother Pharm. 2017;80:999–1004.
Pokorny R, McPherson JP, Haaland B, Grossmann KF, Luckett C, Voorhies BN, et al. Real-world experience with elective discontinuation of PD-1 inhibitors at 1 year in patients with metastatic melanoma. J Immunother Cancer. 2021;9:e001781.
Sadrolashrafi K, Samlowski W. Retreatment of patients with metastatic cutaneous melanoma who relapse after elective checkpoint inhibitor discontinuation after a complete remission. Oncologist. 2023;28:E270–5.
van Zeijl MCT, van den Eertwegh AJM, Wouters MWJM, de Wreede LC, Aarts MJB, van den Berkmortel FWPJ, et al. Discontinuation of anti-PD-1 monotherapy in advanced melanoma—Outcomes of daily clinical practice. Int J Cancer. 2022;150:317–26.
Warner AB, Palmer JS, Shoushtari AN, Goldman DA, Panageas KS, Hayes SA, et al. Long-Term Outcomes and Responses to Retreatment in Patients With Melanoma Treated With PD-1 Blockade. J Clin Oncol. 2020;38:1655–63.
Whitman ED, Scherrer E, Ou W, Krepler C. Outcomes of retreatment with anti-PD-1 monotherapy after response to first course in patients with cutaneous melanoma. Future Oncol. 2020;16:1441–53.
Chiarion-Sileni V, Pigozzo J, Ascierto PA, Simeone E, Maio M, Calabrò L, et al. Ipilimumab retreatment in patients with pretreated advanced melanoma: The expanded access programme in Italy. Br J Cancer. 2014;110:1721–6.
Formozo AA, Gomes JR, Schmerling RA, Buzaid AC. Retrospective analysis of rechallenge with ipilimumab in patients with metastatic melanoma. J Ski Cancer. 2021;2021:5531864.
Lebbé C, Weber JS, Maio M, Neyns B, Harmankaya K, Hamid O, et al. Survival follow-up and ipilimumab retreatment of patients with advanced melanoma who received ipilimumab in prior phase II studies. Ann Oncol. 2014;25:2277–84.
Robert C, Schadendorf D, Messina M, Hodi FS, O’Day S. Efficacy and safety of retreatment with ipilimumab in patients with pretreated advanced melanoma who progressed after initially achieving disease control. Clin Cancer Res. 2013;19:2232–9.
Shah P, Punekar SR, Pavlick AC. Response to immune checkpoint inhibitor rechallenge after high-grade immune related adverse events in patients with advanced melanoma. Melanoma Res. 2021;31:242–8.
Spain L, Walls G, Messiou C, Turajlic S, Gore M, Larkin J. Efficacy and toxicity of rechallenge with combination immune checkpoint blockade in metastatic melanoma: a case series. Cancer Immunol, Immunother. 2017;66:113–7.
Blank CU, Lucas MW, Scolyer RA, van de Wiel BA, Menzies AM, Lopez-Yurda M, et al. Neoadjuvant nivolumab and ipilimumab in resectable stage III melanoma. N Engl J Med. 2024;391:1696-1708.
Van Not OJ, Van Den Eertwegh AJM, Haanen JB, Blank CU, Aarts MJB, Van Breeschoten J, et al. Improving survival in advanced melanoma patients: a trend analysis from 2013 to 2021. EClinicalMedicine. 2024;69:102485.
Acknowledgements
We thank all physicians and data managers who registered patient data in the Dutch Melanoma Treatment Registry.
Funding
The Dutch Institute for Clinical Auditing foundation received a start-up grant from governmental organization The Netherlands Organization for Health Research and Development (ZonMW, project number 836002002) for the Dutch Melanoma Treatment Registry (DMTR). The DMTR is structurally funded by Bristol-Myers Squibb, Merck Sharpe & Dohme, Novartis and Roche Pharma. Roche Pharma stopped funding in 2019 and Pierre Fabre started funding in 2019. For this work no funding was granted.
Author information
Authors and Affiliations
Contributions
EJD: conceptualization, methodology, formal analysis, data interpretation, writing original draft, review and editing, data collection, project administration. HHN: conceptualization, data interpretation, writing original draft, review and editing, data collection, project administration. AJME: data interpretation, review and editing, data collection, project administration. MJB: data interpretation, review and editing, data collection, project administration. MB: data interpretation, review and editing, data collection, project administration. AMK: data interpretation, review and editing, data collection. MMR: data interpretation, review and editing, data collection. CB: data interpretation, review and editing. MJBA: data interpretation, review and editing, data collection, project administration. FWPJB: data interpretation, review and editing, data collection, project administration. CUB: data interpretation, review and editing, data collection, project administration. WAMB: data interpretation, review and editing, data collection, project administration. JWBG: data interpretation, review and editing, data collection, project administration. GAPH: data interpretation, review and editing, data collection, project administration. DP: data interpretation, review and editing, data collection, project administration. RSR: data interpretation, review and editing, data collection, project administration. AMS: data interpretation, review and editing, data collection, project administration. GV: data interpretation, review and editing, data collection, project administration. MWJMW: data interpretation, review and editing, data collection, project administration. EK: data interpretation, review and editing, data collection, project administration. JBH: data interpretation, review and editing, data collection, project administration. AAMV: data interpretation, review and editing, data collection, project administration. KPMS: conceptualization, data interpretation, review and editing, data collection, project administration, supervision.
Corresponding author
Ethics declarations
Competing interests
HHN has advisory/consultancy relations with Bayer, Roche, Johnson&Johnson, and Illumina. AJME has advisory/consultancy relations with Amgen, Bristol Myers Squibb, Ipsen Biopharmaceuticals, Janssen-Cilag BV, Merck, Merck Sharp & Dohme, Novartis, Pfizer Canada and Pierre Fabre Pharmaceuticals. He received a research grant from Bristol-Myers Squibb, paid to institution. CB received research grants from Philips, Bracco, Sensius, all paid to institution. CUB has advisory/consultancy relations with Bristol Myers Squibb, Roche, Merck and Third Rock Ventures. He received research grants from 4SC, Bristol Myers Squibb, Roche, Merck, NanoString Technologies, all paid to institution. He received an individual research grant from Third Rock Ventures. He is co-founder of Signature Oncology and Imagene BV, and has a pending patent (Patent number WO 2021/177822 A1). GAPH received research grants from Bristol-Myers Squibb and Seerave, all paid to institution. DP has advisory/consultancy relations with Novartis and Pierre Fabre Pharmaceuticals. She received a travel grant from Pierre Fabre Pharmaceuticals. EK has advisory/consultancy relations with Delcath, Immunocore and Lilly, and received research grants not related to this paper from Bristol Myers Squibb, Delcath, Novartis, and Pierre-Fabre. Not related to current work and paid to institute. JBH has advisory/consultancy relations with AZ, BioNTech, CureVac, Eisai, Ipsen, Instil Bio, Iovance Bio, MSD, Novartis, Neogene Therapeutics, Roche, Sanofi, Sastra Cell Therapy, Third Rock Ventures, and T-Knife. He received research grants from Amgen, BioNTech, Bristol Myers Squibb, Novartis, Sastra Cell Therapy. He is editor-in-Chief of ESMO Immmuno-Oncology & Technology (IOTECH). AAMV has advisory/consultancy relations with Bristol-Myers Squibb, MSD, Sanofi, Merck, Pierre Fabre, Novartis, Pfizer, Roche, Eisai and Ipsen. KPMS has advisory/consultancy relations with AbbVie, and Sairopa. She received research grants from Bristol-Myers Squibb, Genmab, Philips, Pierre Fabre, and Tigatx, all paid to institution. She does data and safety monitoring for Sairopa. No other competing interests were reported.
Ethics approval and consent to participate
This study was performed in accordance with the Declaration of Helsinki. In compliance with Dutch regulations, research with DMTR data was approved by the medical ethical committee (METC Leiden University Medical Center, 2013) not to be subject to the Medical Research Involving Human Subjects Act, thereby waiving the requirement for informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
van Dijk, E.J., Nienhuis, H.H., van den Eertwegh, A.J.M. et al. Rechallenge of ipilimumab and nivolumab in advanced melanoma patients after previous ipilimumab-based therapy. Br J Cancer 133, 66–75 (2025). https://doi.org/10.1038/s41416-025-03027-z
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41416-025-03027-z