Abstract
Background
Accumulating evidence suggests that deregulation of DNA damage response (DDR) network affects multiple aspects of the immune system. Herein, we tested the hypothesis that DDR-related signals, measured in peripheral blood mononuclear cells (PBMCs) from Head and Neck Squamous Cell Carcinoma (HNSCC) patients, correlate with the response to immune checkpoint inhibitors.
Methods
Oxidative stress and DDR-related signals were evaluated in PBMCs from 26 healthy controls and 50 recurrent/metastatic HNSCC patients at baseline, who participated in a phase II nivolumab trial (NCT03652142). Spatial transcriptomics in three molecularly defined tissue compartments (tumour, leucocyte, macrophage) from biopsies of overlapping cases were also investigated.
Results
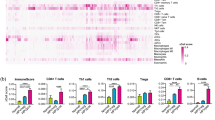
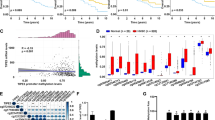
PBMCs from responders to nivolumab therapy showed significantly lower oxidative stress, endogenous DNA damage, DNA repair capacities and apoptosis rates compared with non-responders (all P < 0.04). The analysis of tissue RNA in situ data illustrated that DNA repair pathways showed enrichment in the macrophage compartment of baseline tissue biopsies of responders compared with non-responders (P = 0.049).
Conclusions
Our findings demonstrate that oxidative stress and deregulated DDR-related signals measured in PBMCs from HNSCC patients at baseline correlate with response to nivolumab and, if further validated, may be exploited as novel non-invasive biomarkers and the design of clinical trials.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data relevant to the study are included in the article or uploaded as supplementary information.
References
Nor JE, Gutkind JS. Head and neck cancer in the new era of precision medicine. J Dent Res. 2018;97:601–2.
Prasad V, Kaestner V. Nivolumab and pembrolizumab: monoclonal antibodies against programmed cell death-1 (PD-1) that are interchangeable. Semin Oncol. 2017;44:132–5.
Bauml JM, Aggarwal C, Cohen RB. Immunotherapy for head and neck cancer: where are we now and where are we going? Ann Transl Med. 2019;7:S75.
Harrington KJ, Burtness B, Greil R, Soulieres D, Tahara M, de Castro G Jr, et al. Pembrolizumab with or without chemotherapy in recurrent or metastatic head and neck squamous cell carcinoma: updated results of the phase III KEYNOTE-048 study. J Clin Oncol. 2023;41:790–802.
Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40:179–204.
Pateras IS, Havaki S, Nikitopoulou X, Vougas K, Townsend PA, Panayiotidis MI, et al. The DNA damage response and immune signaling alliance: Is it good or bad? Nature decides when and where. Pharmacol Ther. 2015;154:36–56.
Lee ECY, Kok JST, Teh BT, Lim KS. Interplay between the DNA damage response and immunotherapy response in cancer. Int J Mol Sci. 2022;23.
Nenclares P, Rullan A, Tam K, Dunn LA, St John M, Harrington KJ. Introducing checkpoint inhibitors into the curative setting of head and neck cancers: lessons learned, future considerations. Am Soc Clin Oncol Educ Book. 2022;42:1–16.
Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12:931–47.
Qiu X, Hou QH, Shi QY, Jiang HX, Qin SY. Identification of hub prognosis-associated oxidative stress genes in pancreatic cancer using integrated bioinformatics analysis. Front Genet. 2020;11:595361.
Choudhari SK, Chaudhary M, Gadbail AR, Sharma A, Tekade S. Oxidative and antioxidative mechanisms in oral cancer and precancer: a review. Oral Oncol. 2014;50:10–8.
Stich HF, Anders F. The involvement of reactive oxygen species in oral cancers of betel quid/tobacco chewers. Mutat Res. 1989;214:47–61.
Maj T, Wang W, Crespo J, Zhang H, Wang W, Wei S, et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat Immunol. 2017;18:1332–41.
de Sa Junior, Camara PL, Porcacchia DAD, Fonseca AS, Jorge SD PMM, Araldi RP, et al. The roles of ROS in cancer heterogeneity and therapy. Oxid Med Cell Longev. 2017;2017:2467940.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Vlachogiannis NI, Gatsiou A, Silvestris DA, Stamatelopoulos K, Tektonidou MG, Gallo A, et al. Increased adenosine-to-inosine RNA editing in rheumatoid arthritis. J Autoimmun. 2020;106:102329.
Sfikakis PP, Vlachogiannis NI, Ntouros PA, Mavrogeni S, Maris TG, Karantanas AH, et al. Microvasculopathy-related hemorrhagic tissue deposition of iron may contribute to fibrosis in systemic sclerosis: hypothesis-generating insights from the literature and preliminary findings. Life. 2022;12.
Psyrri A, Gkotzamanidou M, Papaxoinis G, Krikoni L, Economopoulou P, Kotsantis I, et al. The DNA damage response network in the treatment of head and neck squamous cell carcinoma. ESMO Open. 2021;6:100075.
Gavrielatou N, Vathiotis I, Aung TN, Shafi S, Burela S, Fernandez AI, et al. Digital spatial profiling links beta-2-microglobulin expression with immune checkpoint blockade outcomes in head and neck squamous cell carcinoma. Cancer Res Commun. 2023;3:558–63.
Bai S, Feng Q, Pan XY, Zou H, Chen HB, Wang P, et al. Overexpression of wild-type p21Ras plays a prominent role in colorectal cancer. Int J Mol Med. 2017;39:861–8.
Gavrielatou N, Fortis E, Spathis A, Anastasiou M, Economopoulou P, Foukas GRP, et al. B-cell infiltration is associated with survival outcomes following programmed cell death protein 1 inhibition in head and neck squamous cell carcinoma. Ann Oncol. 2024;35:340–50.
Souliotis VL, Vlachogiannis NI, Pappa M, Argyriou A, Ntouros PA, Sfikakis PP. DNA Damage response and oxidative stress in systemic autoimmunity. Int J Mol Sci. 2019;21.
Economopoulou P, Anastasiou M, Papaxoinis G, Spathas N, Spathis A, Oikonomopoulos N, et al. Patterns of response to immune checkpoint inhibitors in association with genomic and clinical features in patients with head and neck squamous cell carcinoma (HNSCC). Cancers. 2021;13.
Mouw KW, Goldberg MS, Konstantinopoulos PA, D’Andrea AD. DNA Damage and Repair Biomarkers of Immunotherapy Response. Cancer Discov. 2017;7:675–93.
Hegde ML, Izumi T, Mitra S. Oxidized base damage and single-strand break repair in mammalian genomes: role of disordered regions and posttranslational modifications in early enzymes. Prog Mol Biol Transl Sci. 2012;110:123–53.
Sliwinski T, Markiewicz L, Rusin P, Kabzinski J, Dziki L, Milonski J, et al. Impaired nucleotide excision repair pathway as a possible factor in pathogenesis of head and neck cancer. Mutat Res. 2011;716:51–8.
Souliotis VL, Vougas K, Gorgoulis VG, Sfikakis PP. Defective DNA repair and chromatin organization in patients with quiescent systemic lupus erythematosus. Arthritis Res Ther. 2016;18:182.
Shammas MA, Shmookler Reis RJ, Koley H, Batchu RB, Li C, Munshi NC. Dysfunctional homologous recombination mediates genomic instability and progression in myeloma. Blood. 2009;113:2290–7.
Choudhury JH, Choudhury B, Kundu S, Ghosh SK. Combined effect of tobacco and DNA repair genes polymorphisms of XRCC1 and XRCC2 influence high risk of head and neck squamous cell carcinoma in northeast Indian population. Med Oncol. 2014;31:67.
Sliwinski T, Walczak A, Przybylowska K, Rusin P, Pietruszewska W, Zielinska-Blizniewska H, et al. Polymorphisms of the XRCC3 C722T and the RAD51 G135C genes and the risk of head and neck cancer in a Polish population. Exp Mol Pathol. 2010;89:358–66.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Raudenska M, Balvan J, Masarik M. Cell death in head and neck cancer pathogenesis and treatment. Cell Death Dis. 2021;12:192.
Pariset E, Bertucci A, Petay M, Malkani S, Lopez Macha A, Paulino Lima IG, et al. DNA damage baseline predicts resilience to space radiation and radiotherapy. Cell Rep. 2020;33:108434.
Araujo BdLV, Hansen M, Spanggaard I, Rohrberg K, Reker Hadrup S, Lassen U, et al. Immune cell profiling of peripheral blood as signature for response during checkpoint inhibition across cancer types. Front Oncol. 2021;11:558248.
Zhang L, Hou L, Wu J, Li C, Hu T, Zhu C, et al. Peripheral blood mononuclear cells (PBMCs), an ideal liquid biopsy approach to evaluate systematic immunity and predict response of neoadjuvant chemo-immunotherapy in resectable NSCLC. J Clin Oncol. 2022;40:e20618–e.
Pallett LJ, Davies J, Colbeck EJ, Robertson F, Hansi N, Easom NJW, et al. IL-2(high) tissue-resident T cells in the human liver: Sentinels for hepatotropic infection. J Exp Med. 2017;214:1567–80.
Smolders J, Heutinck KM, Fransen NL, Remmerswaal EBM, Hombrink P, Ten Berge IJM, et al. Tissue-resident memory T cells populate the human brain. Nat Commun. 2018;9:4593.
Colombo SAP, Brown SL, Hepworth MR, Hankinson J, Granato F, Kitchen SJ, et al. Comparative phenotype of circulating versus tissue immune cells in human lung and blood compartments during health and disease. Discov Immunol. 2023;2:kyad009.
Lucca LE, Axisa PP, Lu B, Harnett B, Jessel S, Zhang L, et al. Circulating clonally expanded T cells reflect functions of tumor-infiltrating T cells. J Exp Med. 2021;218.
Vanguri RS, Smithy JW, Li Y, Zhuang M, Maher CA, Aleynick N, et al. Integration of peripheral blood- and tissue-based biomarkers of response to immune checkpoint blockade in urothelial carcinoma. J Pathol. 2023;261:349–60.
Milkovic L, Cipak Gasparovic A, Cindric M, Mouthuy PA. Short overview of ROS as cell function regulators and their implications in therapy concepts. Cells. 2019;8.
Van Loenhout J, Peeters M, Bogaerts A. Oxidative stress-inducing anticancer therapies: taking a closer look at their immunomodulating effects. Antioxidants. 2020;9.
Barros EM, McIntosh SA, Savage KI. The DNA damage induced immune response: Implications for cancer therapy. DNA Repair. 2022;120:103409.
Kay J, Thadhani E, Samson L, Engelward B. Inflammation-induced DNA damage, mutations and cancer. DNA Repair. 2019;83:102673.
Banissi C, Ghiringhelli F, Chen L, Carpentier AF. Treg depletion with a low-dose metronomic temozolomide regimen in a rat glioma model. Cancer Immunol Immunother. 2009;58:1627–34.
Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–8.
Strickland KC, Howitt BE, Shukla SA, Rodig S, Ritterhouse LL, Liu JF, et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. 2016;7:13587–98.
Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res. 2019;25:3753–8.
Marcus L, Fashoyin-Aje LA, Donoghue M, Yuan M, Rodriguez L, Gallagher PS, et al. FDA approval summary: pembrolizumab for the treatment of tumor mutational burden-high solid tumors. Clin Cancer Res. 2021;27:4685–9.
Farkkila A, Gulhan DC, Casado J, Jacobson CA, Nguyen H, Kochupurakkal B, et al. Immunogenomic profiling determines responses to combined PARP and PD-1 inhibition in ovarian cancer. Nat Commun. 2020;11:1459.
Sharma P, Pachynski RK, Narayan V, Flechon A, Gravis G, Galsky MD, et al. Nivolumab plus ipilimumab for metastatic castration-resistant prostate cancer: preliminary analysis of patients in the CheckMate 650 trial. Cancer Cell. 2020;38:489–99.e3.
Teo MY, Seier K, Ostrovnaya I, Regazzi AM, Kania BE, Moran MM, et al. Alterations in DNA damage response and repair genes as potential marker of clinical benefit from PD-1/PD-L1 blockade in advanced urothelial cancers. J Clin Oncol. 2018;36:1685–94.
Konstantinopoulos PA, Luo W, Liu JF, Gulhan DC, Krasner C, Ishizuka JJ, et al. Phase II study of avelumab in patients with mismatch repair deficient and mismatch repair proficient recurrent/persistent endometrial cancer. J Clin Oncol. 2019;37:2786–94.
Hanna GJ, Lizotte P, Cavanaugh M, Kuo FC, Shivdasani P, Frieden A, et al. Frameshift events predict anti-PD-1/L1 response in head and neck cancer. JCI Insight. 2018;3.
Moutafi M, Economopoulou P, Rimm D, Psyrri A. PARP inhibitors in head and neck cancer: molecular mechanisms, preclinical and clinical data. Oral Oncol. 2021;117:105292.
Moutafi M, Koliou GA. Phase II window study of olaparib alone or with cisplatin or durvalumab in operable head and neck cancer. Cancer Res Commun. 2023;3:1514–23.
Oliva M, Llop S, Vidales Z, Arrazubi V, Baste N, Brana I, et al. TTCC-2022-01: Niraparib and dostarlimab in locally-advanced head and neck squamous cell carcinoma treated with (chemo) radiotherapy (RADIAN). J Clin Oncol. 2024;42:TPS6125–TPS.
Garassino MC, Gadgeel S, Speranza G, Felip E, Esteban E, Dómine M, et al. Pembrolizumab plus pemetrexed and platinum in nonsquamous non–small-cell lung cancer: 5-year outcomes from the phase 3 KEYNOTE-189 study. J Clin Oncol. 2023;41:1992–8.
Paz-Ares LG, Ciuleanu T-E, Cobo M, Bennouna J, Schenker M, Cheng Y, et al. First-line nivolumab plus ipilimumab with chemotherapy versus chemotherapy alone for metastatic NSCLC in CheckMate 9LA: 3-year clinical update and outcomes in patients with brain metastases or select somatic mutations. J Thorac Oncol. 2023;18:204–22.
Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med. 2022;386:449–62.
Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383:1218–30.
Cortes J, Rugo HS, Cescon DW, Im S-A, Yusof MM, Gallardo C, et al. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N Engl J Med. 2022;387:217–26.
Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382:810–21.
Mayer ELGN, Leon-Ferre RA. TBCRC 056: a phase II study of neoadjuvant niraparib with dostarlimab for patients with BRCA- or PALB2-mutated breast cancer: results from the ER+/HER2- cohort. In: Proceedings of the San Antonio Breast Cancer Symposium; San Antonio, TX, December 10–13, 2024 RF3-01.
Harano K, Nakao T, Nishio S, Katsuta T, Tasaki K, Takehara K, et al. Neoadjuvant combination treatment of olaparib and pembrolizumab for patients with HRD-positive advanced ovarian cancer. J Clin Oncol. 2024;42:5545.
Wang Z, Zhao J, Wang G, Zhang F, Zhang Z, Zhang F, et al. Comutations in DNA damage response pathways serve as potential biomarkers for immune checkpoint blockade. Cancer Res. 2018;78:6486–96.
Parkes EE, Walker SM, Taggart LE, McCabe N, Knight LA, Wilkinson R, et al. Activation of STING-dependent innate immune signaling By S-phase-specific DNA damage in breast cancer. J Natl Cancer Inst. 2017;109.
Wang Y, Jiao X, Li S, Chen H, Wei X, Liu C, et al. Alterations in DNA damage response and repair genes as potential biomarkers for immune checkpoint blockade in gastrointestinal cancer. Acta Pharm Sin B. 2021;19:1139–49.
Heath O, Berlato C, Maniati E. Chemotherapy induces tumor-associated macrophages that aid adaptive immune responses in ovarian cancer. Cancer Immunol Res. 2021;9:665–81.
Acknowledgements
Graphical abstract contains items that have been adapted from Servier Medical Art by Servier (https://smart.servier.com), licensed under Creative Commons Attribution 4.0 International License.
Funding
This work was supported from the Hellenic Society of Medical Oncology (HeSMO).
Author information
Authors and Affiliations
Contributions
Conceptualisation: AP, VLS, CP. Methodology: CP, PE, AS, IK, MA, NG, PF, IP, GPF, DR, IL. Investigation: AP, VLS, CP, PE, PF, MM. Visualisation: PE, IK, MA, IL, IP. Resources: AP, PE, DR, MM, AK. Funding acquisition: AP, VLS. Writing original draft: CP, PE. Writing and approval: all authors.
Corresponding author
Ethics declarations
Competing interests
Amanda Psyrri has received honoraria and research funding from BMS, MSD, Roche, Pfizer, Merck Serono. AstraZeneca, Ipsen. She has also received research support from KURA Oncology.
Ethics approval and consent to participate
The study was approved by the Ethical Committee of Attikon Hospital, Athens, Greece. All participants gave informed consent to participate in the study. The study was conducted according to the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Papanikolaou, C., Economopoulou, P., Spathis, A. et al. Association of DNA damage response signals and oxidative stress status with nivolumab efficacy in patients with Head and Neck Squamous Cell Carcinoma. Br J Cancer 133, 353–364 (2025). https://doi.org/10.1038/s41416-025-03032-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41416-025-03032-2