Abstract
Background
This multicenter phase Ib/II trial aimed to evaluate the safety and efficacy of combining durvalumab, tremelimumab, and paclitaxel as second-line treatment for biomarker-selected patients with metastatic gastric cancer.
Methods
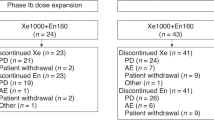
In phase Ib, the standard 3 + 3 dose escalation method was used. Durvalumab and tremelimumab were administered every 4 weeks for 13 and 4 cycles, respectively, combining paclitaxel 80 mg/m2 (dose level 2) or 60 mg/m2 (dose level 1) on days 1, 8, and 15. The primary outcome for phase II was the objective response rate (ORR).
Results:
In phase Ib (n = 7), dose level-1 was selected as the recommended phase II dose. In phase II, 48 patients were enrolled: microsatellite instability-high or deficient mismatch repair protein tumors (n = 16); EBV-positive tumors (n = 15); high tumor mutation burden ( ≥ 5/Mb) (n = 11); CD274 amplification (n = 5); and POLD1 mutation (n = 1). The ORR was 52.1%, meeting the primary endpoint. The median progression-free survival and overall survival were 5.3 and 13.1 months, respectively. The most common any-grade and grade 3–4 adverse events were anemia (41.7%) and neutropenia (10.4%), respectively.
Conclusions
Durvalumab-tremelimumab with paclitaxel was tolerable and efficacious in biomarker-selected gastric cancer patients as a second-line treatment, highlighting the importance of biomarker-based approaches for immunotherapy in gastric cancer.
Clinical trial registration
NCT03751761.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Data are available upon reasonable request to corresponding authors.
Change history
16 June 2025
In this article the author’s name Keun-Wook Lee was incorrectly written as Keun Wook Lee.
The author’s name has been corrected in both the author list and in the equal contributions statement present on the opening page footnote.
20 June 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41416-025-03092-4
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49.
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–71.
Janjigian YY, Ajani JA, Moehler M, Shen L, Garrido M, Gallardo C, et al. First-Line Nivolumab Plus Chemotherapy for Advanced Gastric, Gastroesophageal Junction, and Esophageal Adenocarcinoma: 3-Year Follow-Up of the Phase III CheckMate 649 Trial. J Clin Oncol. 2024;42:2012–2020.
Xu J, Jiang H, Pan Y, Gu K, Cang S, Han L, et al. Sintilimab Plus Chemotherapy for Unresectable Gastric or Gastroesophageal Junction Cancer: The ORIENT-16 Randomized Clinical Trial. Jama. 2023;330:2064–74.
Rha SY, Oh DY, Yañez P, Bai Y, Ryu MH, Lee J, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24:1181–95.
Qiu MZ, Oh DY, Kato K, Arkenau T, Tabernero J, Correa MC, et al. Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first line treatment for advanced gastric or gastro-oesophageal junction adenocarcinoma: RATIONALE-305 randomised, double blind, phase 3 trial. Bmj. 2024;385:e078876.
Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–35.
Sevko A, Michels T, Vrohlings M, Umansky L, Beckhove P, Kato M, et al. Antitumor effect of paclitaxel is mediated by inhibition of myeloid-derived suppressor cells and chronic inflammation in the spontaneous melanoma model. J Immunol. 2013;190:2464–71.
Michels T, Shurin GV, Naiditch H, Sevko A, Umansky V, Shurin MR. Paclitaxel promotes differentiation of myeloid-derived suppressor cells into dendritic cells in vitro in a TLR4-independent manner. J Immunotoxicol. 2012;9:292–300.
Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24:1449–58.
Kulangara K, Zhang N, Corigliano E, Guerrero L, Waldroup S, Jaiswal D, et al. Clinical Utility of the Combined Positive Score for Programmed Death Ligand-1 Expression and the Approval of Pembrolizumab for Treatment of Gastric Cancer. Arch Pathol Lab Med. 2019;143:330–7.
Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21:1353–65.
Yarchoan M, Hopkins A, Jaffee EM. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl J Med. 2017;377:2500–1.
Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51:202–6.
Ma X, Dong L, Liu X, Ou K, Yang L. POLE/POLD1 mutation and tumor immunotherapy. J Exp Clin Cancer Res. 2022;41:216.
Kim HD, Park SH. Immunological and clinical implications of immune checkpoint blockade in human cancer. Arch Pharm Res. 2019;42:567–81.
Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N. Engl J Med. 2019;381:2020–31.
Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl J Med. 2017;377:1345–56.
Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl J Med. 2018;378:1277–90.
Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022;1:EVIDoa2100070.
Kim HD, Ryu MH, Park YS, Lee SY, Moon M, Kang YK. Insertion-deletion rate is a qualitative aspect of the tumor mutation burden associated with the clinical outcomes of gastric cancer patients treated with nivolumab. Gastric Cancer. 2022;25:226–34.
Choi DH, Jang HL, Lim SH, Kim ST, Hong JY, Park SH, et al. Prevalence of KRAS amplification in patients with metastatic cancer: Real-world next-generation sequencing analysis. Pathol Res Pr. 2024;261:155473.
Kim JW, Na HY, Lee S, Kim JW, Suh KJ, Kim SH, et al. Clinical implementation of next-generation sequencing testing and genomically-matched therapy: a real-world data in a tertiary hospital. Sci Rep. 2025;15:2171.
Landrum MJ, Lee JM, Benson M, Brown GR, Chao C, Chitipiralla S, et al. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018;46:D1062–d1067.
Shah MA, Shitara K, Ajani JA, Bang YJ, Enzinger P, Ilson D, et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: the randomized, phase 3 GLOW trial. Nat Med. 2023;29:2133–41.
Shitara K, Lordick F, Bang YJ, Enzinger P, Ilson D, Shah MA, et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial. Lancet. 2023;401:1655–68.
Wainberg ZA, Enzinger PC, Kang YK, Qin S, Yamaguchi K, Kim IH, et al. Bemarituzumab in patients with FGFR2b-selected gastric or gastro-oesophageal junction adenocarcinoma (FIGHT): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 2022;23:1430–40.
Lee CK, Kim HS, Jung M, Kim H, Bae WK, Koo DH, et al. Open-Label, Multicenter, Randomized, Biomarker-Integrated Umbrella Trial for Second-Line Treatment of Advanced Gastric Cancer: K-Umbrella Gastric Cancer Study. J Clin Oncol. 2024;42:348–57.
Lee CK, Lee JB, Park SJ, Che J, Kwon WS, Kim HS, et al. Second-line chemoimmunotherapy with nivolumab and paclitaxel in immune-related biomarker-enriched advanced gastric cancer: a multicenter phase Ib/II study. Gastric Cancer. 2024;27:118–30.
Zhao JJ, Yap DWT, Chan YH, Tan BKJ, Teo CB, Syn NL, et al. Low Programmed Death-Ligand 1-Expressing Subgroup Outcomes of First-Line Immune Checkpoint Inhibitors in Gastric or Esophageal Adenocarcinoma. J Clin Oncol. 2022;40:392–402.
Muro K, Kawakami H, Kadowaki S, Makiyama A, Tsuda M, Hirata K, et al. 1513MO A phase II study of nivolumab plus low dose ipilimumab as first-line therapy in patients with advanced gastric or esophago-gastric junction MSI-H tumor: First results of the NO LIMIT study (WJOG13320G/CA209-7W7). Ann Oncol. 2023;34:S852–S853.
Kowanetz M, Zou W, Gettinger SN, Koeppen H, Kockx M, Schmid P, et al. Differential regulation of PD-L1 expression by immune and tumor cells in NSCLC and the response to treatment with atezolizumab (anti-PD-L1). Proc Natl Acad Sci USA. 2018;115:E10119–e10126.
Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9.
Shitara K, Özgüroğlu M, Bang Y-J, Di Bartolomeo M, Mandalà M, Ryu M-h, et al. The association of tissue tumor mutational burden (tTMB) using the Foundation Medicine genomic platform with efficacy of pembrolizumab versus paclitaxel in patients (pts) with gastric cancer (GC) from KEYNOTE-061. J Clin Oncol. 2020;38:4537.
Lee KW, Van Cutsem E, Bang YJ, Fuchs CS, Kudaba I, Garrido M, et al. Association of Tumor Mutational Burden with Efficacy of Pembrolizumab±Chemotherapy as First-Line Therapy for Gastric Cancer in the Phase III KEYNOTE-062 Study. Clin Cancer Res. 2022;28:3489–98.
Vokes NI, Liu D, Ricciuti B, Jimenez-Aguilar E, Rizvi H, Dietlein F, et al. Harmonization of Tumor Mutational Burden Quantification and Association With Response to Immune Checkpoint Blockade in Non-Small-Cell Lung Cancer. JCO Precis Oncol. 2019;3. https://doi.org/10.1038/s41443-021-00452-5.
Nakajima TE, Kadowaki S, Minashi K, Nishina T, Yamanaka T, Hayashi Y, et al. Multicenter Phase I/II Study of Nivolumab Combined with Paclitaxel Plus Ramucirumab as Second-line Treatment in Patients with Advanced Gastric Cancer. Clin Cancer Res. 2021;27:1029–36.
Kim HD, Shin J, Hyung J, Lee H, Moon M, Ma J, et al. Survival outcomes of patients with gastric cancer treated with first-line nivolumab plus chemotherapy based on claudin 18.2 expression. Gastric Cancer. 2025;28:74–82.
Wong JSL, Kwok GGW, Tang V, Li BCW, Leung R, Chiu J, et al. Ipilimumab and nivolumab/pembrolizumab in advanced hepatocellular carcinoma refractory to prior immune checkpoint inhibitors. J Immunother Cancer 2021;9:e001945.
Zimmer L, Apuri S, Eroglu Z, Kottschade LA, Forschner A, Gutzmer R, et al. Ipilimumab alone or in combination with nivolumab after progression on anti-PD-1 therapy in advanced melanoma. Eur J Cancer. 2017;75:47–55.
Gul A, Stewart TF, Mantia CM, Shah NJ, Gatof ES, Long Y, et al. Salvage Ipilimumab and Nivolumab in Patients With Metastatic Renal Cell Carcinoma After Prior Immune Checkpoint Inhibitors. J Clin Oncol. 2020;38:3088–94.
Funding
Study drugs were provided by AstraZeneca (durvalumab and tremelimumab). The research was funded by the National Research and Development Program for Cancer Control (grant number HA17C0054) of the Ministry of Health and Welfare, Republic of Korea, and supported by National Cancer Center (NCC, Korea) Grants 24H1660 and 24H1710.
Author information
Authors and Affiliations
Contributions
Conception and Design: DYZ, MHR and HK; Study materials: KWL, DYZ, HDK, JWK, BJK, YKK, MHR and HK; Collection and assembly: KWL, DYZ, HDK, JWK, BJK, YKK, MHR and HK; Data analysis: KWL, DYZ, HDK, MHR and HK; Manuscript writing: KWL, DYZ, HDK, MHR and HK; Final approval: KWL, DYZ, HDK, JWK, BJK, YKK, MHR and HK; and Supervision: MHR and HK.
Corresponding authors
Ethics declarations
Competing interests
Nothing directly related to this work. Outside of this work, LKW reported receiving research funding for clinical trials from MSD, AstraZeneca, Ono Pharmaceutical, Roche, Merck KGaA, BeiGene, Astellas Pharma, Amgen, Daiichi Sankyo, ALX Oncology, Leap Therapeutics, GlaxoSmithKline, Macrogenics, Taiho Pharmaceutical, Seagen, Y-BIOLOGICS, Bolt Biotherapeutics, Trishula Therapeutics, InventisBio, MedPacto, Ildong Pharmaceutical, Genome & Company, Arcus Biosciences, Elevar Therapeutics, Jazz Pharmaceuticals, TRIO Oncology, Exelixis, IgM Biosciences, Panolos Bioscience, Metafines, Wellmarker Bio, Medicenna, and Erasca. He also received consulting fees from Daiichi Sankyo, MSD, Astellas Pharma, AbbVie, and Metafines; honoraria for lectures or presentations from Sanofi/Aventis, Astellas Pharma, Bayer, Daiichi Sankyo, and Merck KGaA; and has an uncompensated role with ALX Oncology on a data safety monitoring or advisory board. YKK has a consulting or advisory role with ALX Oncology, Amgen, Blueprint Medicines, Bristol-Myers Squibb, DAEHWA Pharmaceutical, Macrogenics, Novartis, Surface Oncology, and Zymeworks. MHR received honoraria from DAEHWA Pharmaceuticals, Bristol Myers Squibb, Lilly, Ono Pharmaceuticals, MSD, Taiho Pharmaceuticals, Novartis, Daiichi Sankyo, and AstraZeneca; and served as a consultant for DAEHWA Pharmaceuticals, Bristol Myers Squibb, Lilly, and Ono Pharmaceuticals. HDK received research grants from Roche/Genentech and AstraZeneca and honoraria from AstraZeneca, Bristol Myers Squibb, Ono Pharmaceuticals, Boryung Pharmaceuticals, MSD, Daiichi Sankyo, Astellas, Boostimmune, and MustBio.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of each participating center. All patients provided written informed consent. This study was performed in accordance with the ethical standards of the Institutional Research Committee and the latest Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, KW., Zang, D.Y., Kim, HD. et al. Multicenter phase Ib/II study of second-line durvalumab and tremelimumab in combination with paclitaxel in patients with biomarker-selected metastatic gastric cancer. Br J Cancer 133, 208–215 (2025). https://doi.org/10.1038/s41416-025-03052-y
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41416-025-03052-y