Abstract
Background
In cancer cachexia, cytokine release by tumours causes weight loss, decreased therapeutic efficacy, and worsened prognosis. Tumour necrosis factor-like weak inducer of apoptosis (TWEAK) and fibroblast inducible factor 14 (Fn14) are cancer cachexia-related proteins; however, their expression in oesophageal squamous cell carcinoma (ESCC) and association with therapeutic resistance remain unclear.
Methods
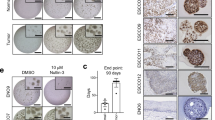
We evaluated how Fn14 knockdown and overexpression in ESCC lines (TE6 and TE10) contributed to proliferation, migration, and chemotherapy resistance in vitro and in vivo. In 135 ESCC patients who underwent esophagectomy after neoadjuvant chemotherapy, tumour expression of TWEAK and Fn14 was evaluated immunohistochemically to assess the association with clinicopathological factors and prognosis, including chemotherapeutic efficacy.
Results
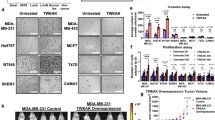
Proliferation, migration, and chemotherapy resistance of ESCC cell lines were decreased by Fn14 knockdown but increased by Fn14 overexpression. Patients with Fn14-overexpressing ESCC had a decreased response rate to neoadjuvant chemotherapy and significantly lower rates of overall and recurrence-free survival, while concurrent expression of TWEAK and Fn14 was associated with further reductions in the response rate to chemotherapy and the rates of overall and recurrence-free survival.
Conclusions
TWEAK and Fn14 expression was associated with treatment resistance and prognosis in ESCC. Inhibiting the TWEAK/Fn14 axis may reduce treatment resistance and improve prognosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are not publicly available because they contain private information pertaining to the research participants but are available on request from the corresponding author.
References
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–95.
Daas SI, Rizeq BR, Nasrallah GK. Adipose tissue dysfunction in cancer cachexia. J Cell Physiol. 2018;234:13–22.
Webster JM, Kempen L, Hardy RS, Langen RCJ. Inflammation and skeletal muscle wasting during cachexia. Front Physiol. 2020;11:597675.
Ross PJ, Ashley S, Norton A, Priest K, Waters JS, Eisen T, et al. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br J Cancer. 2004;90:1905–11.
Keigo N, Koichiro H, Teppei K, Junji T, Yuichi N, Hironori O, et al. Usefulness of the cachexia index as a prognostic indicator for patients with gastric cancer. Ann Gastroenterol Surg. 2023;7:733–40.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Primers. 2018;4:17105.
Junya O, Soji O, Koshiro I, Hiroyuki D. Clinical significance of sarcopenic dysphagia for patients with esophageal cancer undergoing esophagectomy: a review. Ann Gastroenterol Surg. 2022;6:738–45.
Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681–92.
Miyata H, Yoshioka A, Yamasaki M, Nushijima Y, Takiguchi S, Fujiwara Y, et al. Tumor budding in tumor invasive front predicts prognosis and survival of patients with esophageal squamous cell carcinomas receiving neoadjuvant chemotherapy. Cancer. 2009;115:3324–34.
Kitagawa Y, Uno T, Oyama T, Kato K, Kato H, Kawakubo H, et al. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1. Esophagus. 2019;16:1–24.
Temel JS, Abernethy AP, Currow DC, Friend J, Duus EM, Yan Y, et al. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol. 2016;17:519–31.
Currow D, Temel JS, Abernethy A, Milanowski J, Friend J, Fearon KC. ROMANA 3: a phase 3 safety extension study of anamorelin in advanced non-small-cell lung cancer (NSCLC) patients with cachexia. Ann Oncol. 2017;28:1949–56.
Hamauchi S, Furuse J, Takano T, Munemoto Y, Furuya K, Baba H, et al. A multicenter, open-label, single-arm study of anamorelin (ONO-7643) in advanced gastrointestinal cancer patients with cancer cachexia. Cancer. 2019;125:4294–302.
Katakami N, Uchino J, Yokoyama T, Naito T, Kondo M, Yamada K, et al. Anamorelin (ONO-7643) for the treatment of patients with non-small cell lung cancer and cachexia: Results from a randomized, double-blind, placebo-controlled, multicenter study of Japanese patients (ONO-7643-04). Cancer. 2018;124:606–16.
Johnston AJ, Murphy KT, Jenkinson L, Laine D, Emmrich K, Faou P, et al. Targeting of Fn14 prevents cancer-induced cachexia and prolongs survival. Cell. 2015;162:1365–78.
Salzmann S, Seher A, Trebing J, Weisenberger D, Rosenthal A, Siegmund D, et al. Fibroblast growth factor inducible (Fn14)-specific antibodies concomitantly display signaling pathway-specific agonistic and antagonistic activity. J Biol Chem. 2013;288:13455–66.
Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–81.
Varfolomeev E, Goncharov T, Maecker H, Zobel K, Komuves LG, Deshayes K, et al. Cellular inhibitors of apoptosis are global regulators of NF-kappaB and MAPK activation by members of the TNF family of receptors. Sci Signal. 2012;5:ra22.
Vince JE, Silke J. TWEAK shall inherit the earth. Cell Death Differ. 2006;13:1842–4.
Liang L, Cheng C, Hu G, Wang X, Liu J, Yan Z, et al. TWEAK promotes the proliferation of squamous cell carcinoma cells through activating cIAP1 signals. Front Oncol. 2020;10:439.
Yamasaki M, Yasuda T, Yano M, Hirao M, Kobayashi K, Fujitani K, et al. Multicenter randomized phase II study of cisplatin and fluorouracil plus docetaxel (DCF) compared with cisplatin and fluorouracil plus Adriamycin (ACF) as preoperative chemotherapy for resectable esophageal squamous cell carcinoma (OGSG1003). Ann Oncol. 2017;28:116–20.
Sugimura K, Yamasaki M, Yasuda T, Yano M, Hirao M, Fujitani K, et al. Long-term results of a randomized controlled trial comparing neoadjuvant Adriamycin, cisplatin, and 5-fluorouracil vs docetaxel, cisplatin, and 5-fluorouracil followed by surgery for esophageal cancer (OGSG1003). Ann Gastroenterol Surg. 2021;5:75–82.
Willis AL, Tran NL, Chatigny JM, Charlton N, Vu H, Brown SA, et al. The fibroblast growth factor-inducible 14 receptor is highly expressed in HER2-positive breast tumors and regulates breast cancer cell invasive capacity. Mol Cancer Res. 2008;6:725–34.
Tran NL, McDonough WS, Donohue PJ, Winkles JA, Berens TJ, Ross KR, et al. The human Fn14 receptor gene is up-regulated in migrating glioma cells in vitro and overexpressed in advanced glial tumors. Am J Pathol. 2003;162:1313–21.
Aye L, Song X, Yang J, Hu L, Sun X, Zhou J, et al. Identification of a costimulatory molecule gene signature to predict survival and immunotherapy response in head and neck squamous cell carcinoma. Front Cell Dev Biol. 2021;9:695533.
Li N, Hu WJ, Shi J, Xue J, Guo WX, Zhang Y, et al. Roles of fibroblast growth factor-inducible 14 in hepatocellular carcinoma. Asian Pac J Cancer Prev. 2013;14:3509–14.
Li X, Zhu W, Chen Z, Luo L, Huang J, Zhang F, et al. Fibroblast growth factor-inducible 14 regulates cell growth and multidrug resistance of small-cell lung cancer through the nuclear factor-kappaB pathway. Anticancer Drugs. 2014;25:1152–64.
Hersh DS, Harder BG, Roos A, Peng S, Heath JE, Legesse T, et al. The TNF receptor family member Fn14 is highly expressed in recurrent glioblastoma and in GBM patient-derived xenografts with acquired temozolomide resistance. Neuro Oncol. 2018;20:1321–30.
Kwon OH, Kim JH, Kim SY, Kim YS. TWEAK/Fn14 signaling mediates gastric cancer cell resistance to 5-fluorouracil via NF-κB activation. Int J Oncol. 2014;44:583–90.
Wang CY, Cusack JC Jr, Liu R, Baldwin AS Jr. Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nat Med. 1999;5:412–7.
Biton S, Ashkenazi A. NEMO and RIP1 control cell fate in response to extensive DNA damage via TNF-a feedforward signaling. Cell. 2011;145:92–103.
Whitsett TG, Mathews IT, Cardone MH, Lena RJ, Pierceall WE, Bittner M, et al. Mcl-1 mediates TWEAK/Fn14-induced non-small cell lung cancer survival and therapeutic response. Mol Cancer Res. 2014;12:550–9.
Lam ET, Eckhardt SG, Messersmith W, Jimeno A, O’Bryant CL, Ramanathan RK, et al. Phase I study of enavatuzumab, a first-in-class humanized monoclonal antibody targeting the TWEAK receptor, in patients with advanced solid tumors. Mol Cancer Ther. 2018;17:215–21.
Maecker H, Varfolomeev E, Kischkel F, Lawrence D, LeBlanc H, Lee W, et al. TWEAK attenuates the transition from innate to adaptive immunity. Cell. 2005;123:931–44.
Liu C, He H, Li X, Su MA, Cao Y. Dynamic metrics-based biomarkers to predict responders to anti-PD-1 immunotherapy. Br J Cancer. 2019;120:346–55.
Chicheportiche Y, Bourdon P, Xu H, Hsu Y, Scott H, Hession C, et al. TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem. 1997;272:32401–10.
Bodmer JL, Schneider P, Tschopp J. The molecular architecture of the TNF superfamily. Trends Biochem Sci. 2002;27:19–26.
Zaitseva O, Hoffmann A, Otto C, Wajant H. Targeting fibroblast growth factor (FGF)-inducible 14 (Fn14) for tumor therapy. Front Pharmacol. 2022;13:935086.
Pascoe AL, Johnston AJ, Murphy RM. Controversies in TWEAK-Fn14 signaling in skeletal muscle atrophy and regeneration. Cell Mol Life Sci. 2020;77:3369–81.
Acknowledgements
We would like to thank Zenis (https://www.zenis.co.jp) for English language editing.
Funding
This research was partially supported by Japan society for the promotion of science (JSPS) (#. 22K08771).
Author information
Authors and Affiliations
Contributions
Conceptualization and design– KY, TT, YK. Sample and data collection– KA, YK, KM. Data curation and statistical analyses –KA, TS, KT, TM. Supervision– KN, HE, YD. Writing-Original draft– KA. Writing-review and editing– KY, KY.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study had a retrospective design and was approved by the Institutional Ethics Review Committee of Certificate Number 08226-10. All the procedures were performed in accordance with the principles of the Declaration of Helsinki and its amendments.
Consent for publication
Written informed consent was obtained from the patient for publication of these data.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Adachi, K., Yamashita, K., Kamakura, Y. et al. High expression of the cachexia-related protein Fn14 in esophageal squamous cell carcinoma correlates with poor chemotherapy response and anti-Fn14 therapy decreases chemotherapeutic resistance. Br J Cancer 133, 771–783 (2025). https://doi.org/10.1038/s41416-025-03087-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41416-025-03087-1