Abstract
Background
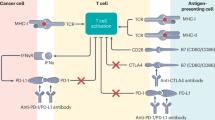
Lung immune prognostic index (LIPI) is associated with survival outcomes in patients with non-small cell lung cancer (NSCLC) receiving immunotherapy, but the association with the occurrence of checkpoint inhibitor pneumonitis (CIP) is unclear.
Methods
We retrospectively included 1824 patients with advanced NSCLC who received immune checkpoint inhibitors (ICIs) at two institutions. Cox regression analysis and cumulative incidence curve were used to evaluate the predictive value of LIPI. Additionally, we performed competing risk analysis using Fine-Gray regression and cumulative incidence curves.
Results
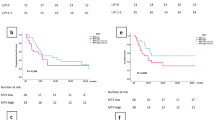
During a median follow-up of 15 months, 99 patients developed CIP. Compared with the good LIPI group, the intermediate LIPI group (HR 1.87, P = 0.007) and poor LIPI group (HR 4.39, P < 0.001) had a higher risk of CIP. Furthermore, we found LIPI was an independent predictor for intermediate-grade CIP (intermediate LIPI: HR 2.11, P = 0.056; poor LIPI: HR 4.51, P = 0.002) and high-grade CIP (intermediate LIPI: HR 6.94, P = 0.014; poor LIPI: HR 44.01, P < 0.001), but not for low-grade CIP (intermediate LIPI: HR 1.31, P = 0.392; poor LIPI: HR 0.72, P = 0.656). Similar results were obtained after competing risk analysis.
Conclusions
LIPI grade shows potential in predicting the risk of CIP during immunotherapy and could be valuable in clinical management.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33.
Bareschino MA, Schettino C, Rossi A, Maione P, Sacco PC, Zeppa R, et al. Treatment of advanced non small cell lung cancer. J Thorac Dis. 2011;3:122–33.
Remon J, Passiglia F, Ahn MJ, Barlesi F, Forde PM, Garon EB, et al. Immune checkpoint inhibitors in thoracic malignancies: review of the existing evidence by an IASLC expert panel and recommendations. J Thorac Oncol. 2020;15:914–47.
Fukihara J, Sakamoto K, Koyama J, Ito T, Iwano S, Morise M, et al. Prognostic impact and risk factors of immune-related pneumonitis in patients with non-small-cell lung cancer who received programmed death 1 inhibitors. Clin Lung Cancer. 2019;20:442–450.e444.
Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest. 2017;151:193–203.
Khunger M, Rakshit S, Pasupuleti V, Hernandez AV, Mazzone P, Stevenson J, et al. Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer: a systematic review and meta-analysis of trials. Chest. 2017;152:271–81.
De Velasco G, Je Y, Bossé D, Awad MM, Ott PA, Moreira RB, et al. Comprehensive meta-analysis of key immune-related adverse events from CTLA-4 and PD-1/PD-L1 inhibitors in cancer patients. Cancer Immunol Res. 2017;5:312–8.
Suresh K, Voong KR, Shankar B, Forde PM, Ettinger DS, Marrone KA, et al. Pneumonitis in non-small cell lung cancer patients receiving immune checkpoint immunotherapy: incidence and risk factors. J Thorac Oncol. 2018;13:1930–9.
Cathcart-Rake EJ, Sangaralingham LR, Henk HJ, Shah ND, Riaz IB, Mansfield AS. A population-based study of immunotherapy-related toxicities in lung cancer. Clin Lung Cancer. 2020;21:421–427.e422.
Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26:2375–91.
Yang J, Lyu M, Feng X, Liu F, Zeng R, Sun X, et al. The predict factors and clinical prognosis value of immune-related pneumonia of receiving PD-1 inhibitor in advanced non-small cell lung cancer: a retrospective study. Int Immunopharmacol. 2024;142:113140.
Ngamphaiboon N, Ithimakin S, Siripoon T, Sintawichai N, Sriuranpong V. Patterns and outcomes of immune-related adverse events in solid tumor patients treated with immune checkpoint inhibitors in Thailand: a multicenter analysis. BMC Cancer. 2021;21:1275.
Mitropoulou G, Daccord C, Sauty A, Pasche A, Egger B, Aedo Lopez V, et al. Immunotherapy-induced airway disease: a new pattern of lung toxicity of immune checkpoint inhibitors. Respiration. 2020;99:181–6.
Chu X, Zhao J, Zhou J, Zhou F, Jiang T, Jiang S, et al. Association of baseline peripheral-blood eosinophil count with immune checkpoint inhibitor-related pneumonitis and clinical outcomes in patients with non-small cell lung cancer receiving immune checkpoint inhibitors. Lung Cancer. 2020;150:76–82.
Charrier M, Mezquita L, Lueza B, Dupraz L, Planchard D, Remon J, et al. Circulating innate immune markers and outcomes in treatment-naïve advanced non-small cell lung cancer patients. Eur J Cancer. 2019;108:88–96.
Sorich MJ, Rowland A, Karapetis CS, Hopkins AM. Evaluation of the lung immune prognostic index for prediction of survival and response in patients treated with atezolizumab for NSCLC: pooled analysis of clinical trials. J Thorac Oncol. 2019;14:1440–6.
Ruiz-Bañobre J, Areses-Manrique MC, Mosquera-Martínez J, Cortegoso A, Afonso-Afonso FJ, de Dios-Álvarez N, et al. Evaluation of the lung immune prognostic index in advanced non-small cell lung cancer patients under nivolumab monotherapy. Transl Lung Cancer Res. 2019;8:1078–85.
Riedl JM, Barth DA, Foris V, Posch F, Mollnar S, Stotz M, et al. 1263P - External validation and longitudinal extension of the LIPI (Lung Immune Prognostic Index) for immunotherapy outcomes in advanced non-small cell lung cancer. Ann Oncol. 2019;30:v514.
Kazandjian D, Gong Y, Keegan P, Pazdur R, Blumenthal GM. Prognostic value of the lung immune prognostic index for patients treated for metastatic non-small cell lung cancer. JAMA Oncol. 2019;5:1481–5.
Park HY, Kang D, Shin SH, Yoo KH, Rhee CK, Suh GY, et al. Chronic obstructive pulmonary disease and lung cancer incidence in never smokers: a cohort study. Thorax. 2020;75:506–9.
Kilic A, Mathier MA, Hickey GW, Sultan I, Morell VO, Mulukutla SR, et al. Evolving trends in adult heart transplant with the 2018 heart allocation policy change. JAMA Cardiol. 2021;6:159–67.
Fine J, Gray R. A porportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509.
Ghanbar MI, Suresh K. Pulmonary toxicity of immune checkpoint immunotherapy. J Clin Invest. 2024:134:e170503.
Zhang M, Fan Y, Nie L, Wang G, Sun K, Cheng Y. Clinical outcomes of immune checkpoint inhibitor therapy in patients with advanced non-small cell lung cancer and preexisting interstitial lung diseases: a systematic review and meta-analysis. Chest. 2022;161:1675–86.
Fujisawa Y, Yoshino K, Otsuka A, Funakoshi T, Fujimura T, Yamamoto Y, et al. Fluctuations in routine blood count might signal severe immune-related adverse events in melanoma patients treated with nivolumab. J Dermatol Sci. 2017;88:225–31.
Diehl A, Yarchoan M, Hopkins A, Jaffee E, Grossman SA. Relationships between lymphocyte counts and treatment-related toxicities and clinical responses in patients with solid tumors treated with PD-1 checkpoint inhibitors. Oncotarget. 2017;8:114268–80.
Matsukane R, Watanabe H, Minami H, Hata K, Suetsugu K, Tsuji T, et al. Continuous monitoring of neutrophils to lymphocytes ratio for estimating the onset, severity, and subsequent prognosis of immune related adverse events. Sci Rep. 2021;11:1324.
Zoghbi, M, Patel, BA, Roulleaux Dugage, M, Mezquita, L, Bahleda, R, Dufresne, A et al. Association of Lung Immune Prognostic Index (LIPI) with disease control rate and progression-free survival in patients with soft-tissue sarcoma treated with immunotherapy in early-phase trials. Cancers. 2024;16:4053.
Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 2018;4:351–7.
Caramori G, Ruggeri P, Mumby S, Ieni A, Lo Bello F, Chimankar V, et al. Molecular links between COPD and lung cancer: new targets for drug discovery? Expert Opin Ther Targets. 2019;23:539–53.
Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–68.
Diem S, Kasenda B, Spain L, Martin-Liberal J, Marconcini R, Gore M, et al. Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-PD-1 therapy in metastatic melanoma. Br J Cancer. 2016;114:256–61.
Kargl J, Busch SE, Yang GH, Kim KH, Hanke ML, Metz HE, et al. Neutrophils dominate the immune cell composition in non-small cell lung cancer. Nat Commun. 2017;8:14381.
Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16:431–46.
Lin MX, Zang D, Liu CG, Han X, Chen J. Immune checkpoint inhibitor-related pneumonitis: research advances in prediction and management. Front Immunol. 2024;15:1266850.
Funding
This study was supported by grants from the Major Program of Special Project for Technology Innovation of Hubei Province (2023BCB014), the National Natural Science Foundation of China (82172034, 82272083, 82472058) and the Fundamental Research Funds for the Central Universities (20242422).
Author information
Authors and Affiliations
Contributions
Bingxin Gong and Yusheng Guo drafted the manuscript; Qi Wan and Yi Li revised the manuscript; Bingxin Gong and Jie Lou performed data analysis and interpretation; Guisheng Zhang and Qi Wan collected the data; Lingli Li and Lian Yang made substantial contributions to the conception and design of the work. All authors involved in manuscript writing and final approval of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations. This study was approved by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology (No. S048) and The First Affiliated Hospital of Guangzhou Medical University (ES-2024-K173-01), and informed consent was obtained from all participants. Written informed consent for publication of the images has been obtained from the authors.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gong, B., Guo, Y., Wan, Q. et al. The lung immune prognostic index stratifies the occurrence of checkpoint inhibitor pneumonitis in advanced non-small cell lung cancer patients: a multi-institutional cohort study. Br J Cancer (2025). https://doi.org/10.1038/s41416-025-03124-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41416-025-03124-z