Abstract
Background
Neurofibromatosis type 1 (NF1) is an autosomal dominant disorder characterized by neurofibromas, with 5–13% of patients risk developing malignant peripheral nerve sheath tumors (MPNST). Current treatments for MPNST are largely ineffective. AXL, overexpressed in MPNST, is a potential target for Chimeric Antigen Receptor T (CAR-T) cell therapy. This study evaluates the immunophenotypes, efficacy, and safety of NF1-derived AXL-CAR-T cells in treating MPNST.
Methods
AXL-CAR-T cells, containing an anti-AXL single-chain variable fragment, were derived from NF1 patients (n = 27) and healthy donors (n = 15). Immunophenotypes were characterized using CCR7, CD45RA, CD4, and CD8 markers. The cytotoxicity of CAR-T cells was tested in vitro against MPNST cells, and efficacy and safety were evaluated in an MPNST xenograft mouse model. Multiplex immunoassays and ELISA measured cytokines and granzyme b release.
Results
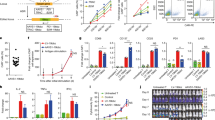
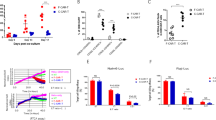
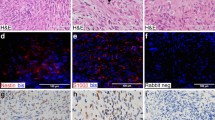
AXL-CAR-T cells from both NF1 patients and healthy donors had a similar partition in stem cell-like memory T cell composition and comparable ex vivo expansion. AXL-CAR-T cells from both groups effectively lysed MPNST cells in vitro. In vivo, tumor volumes in xenograft mice were significantly reduced with no on-target off-tumor toxicity.
Conclusions
NF1 patient-derived AXL-CAR-T cells demonstrated similar quality and efficacy to those from healthy donors, supporting their potential autologous therapy for MPNST.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All the data relevant to this study are included in the article. Further inquiries can be directed to the corresponding author.
References
Zhu Y, Ghosh P, Charnay P, Burns DK, Parada LF. Neurofibromas in NF1: Schwann cell origin and role of tumor environment. Science. 2002;296:920–2.
Friedman JM, Birch PH. Type 1 neurofibromatosis: a descriptive analysis of the disorder in 1,728 patients. Am J Med Genet. 1997;70:138–43.
Lee MJ, Stephenson DA. Recent developments in neurofibromatosis type 1. Curr Opin Neurol. 2007;20:135–41.
Gutmann DH, Ferner RE, Listernick RH, Korf BR, Wolters PL, Johnson KJ. Neurofibromatosis type 1. Nat Rev Dis Primers. 2017;3:17004.
Korf BR. Plexiform neurofibromas. Am J Med Genet. 1999;89:31–7.
Evans D, Baser M, McGaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet. 2002;39:311–4.
Hirose T, Scheithauer BW, Sano T. Perineurial malignant peripheral nerve sheath tumor (MPNST): a clinicopathologic, immunohistochemical, and ultrastructural study of seven cases. Am J Surg Pathol. 1998;22:1368–78.
Hruban RH, Shiu MH, Senie RT, Woodruff JM. Malignant peripheral nerve sheath tumors of the buttock and lower extremity. A study of 43 cases. Cancer. 1990;66:1253–65.
Jackson HJ, Rafiq S, Brentjens RJ. Driving CAR T-cells forward. Nat Rev Clin Oncol. 2016;13:370–83.
Park JH, Geyer MB, Brentjens RJ. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood. 2016;127:3312–20.
Park JH, Riviere I, Gonen M, Wang X, Senechal B, Curran KJ, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378:449–59.
Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak O, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377:2545–54.
Criscitiello C. Tumor-associated antigens in breast cancer. Breast Care. 2012;7:262–6.
Zhu G, Zhang Q, Zhang J, Liu F. Targeting tumor-associated antigen: a promising CAR-T therapeutic strategy for glioblastoma treatment. Front Pharmacol. 2021;12:661606.
Golinelli G, Grisendi G, Prapa M, Bestagno M, Spano C, Rossignoli F, et al. Targeting GD2-positive glioblastoma by chimeric antigen receptor empowered mesenchymal progenitors. Cancer Gene Ther. 2020;27:558–70.
Weldon JE, Xiang L, Zhang J, Beers R, Walker DA, Onda M, et al. A recombinant immunotoxin against the tumor-associated antigen mesothelin reengineered for high activity, low off-target toxicity, and reduced antigenicity. Mol Cancer Ther. 2013;12:48–57.
Graham DK, DeRyckere D, Davies KD, Earp HS. The TAM family: phosphatidylserine-sensing receptor tyrosine kinases gone awry in cancer. Nat Rev Cancer. 2014;14:769–85.
Cruz VH, Arner EN, Du W, Bremauntz AE, Brekken RA. Axl-mediated activation of TBK1 drives epithelial plasticity in pancreatic cancer. JCI Insight. 2019;5:e126117.
Gay CM, Balaji K, Byers LA. Giving AXL the axe: targeting AXL in human malignancy. Brit J Cancer. 2017;116:415–23.
Paccez JD, Vogelsang M, Parker MI, Zerbini LF. The receptor tyrosine kinase Axl in cancer: biological functions and therapeutic implications. Int J Cancer. 2014;134:1024–33.
Davidsen KT, Haaland GS, Lie MK, Lorens JB, Engelsen AST. The role of Axl receptor tyrosine kinase in tumor cell plasticity and therapy resistance. In: Akslen LA, Watnick RS, editors. Biomarkers of the tumor microenvironment: basic studies and practical applications. Cham: Springer International Publishing; 2017. p. 351–76.
Johansson G, Peng P-C, Huang P-Y, Chien H-F, Hua K-T, Kuo M-L, et al. Soluble AXL: a possible circulating biomarker for neurofibromatosis type 1 related tumor burden. PloS One. 2014;9:e115916.
Torres KE, Liu J, Young E, Huang KL, Ghadimi M, Lusby K, et al. Expression of ‘drugable’ tyrosine kinase receptors in malignant peripheral nerve sheath tumour: potential molecular therapeutic targets for a chemoresistant cancer. Histopathology. 2011;59:156–9.
Chen F, Song Q, Yu Q. Axl inhibitor R428 induces apoptosis of cancer cells by blocking lysosomal acidification and recycling independent of Axl inhibition. Am J Cancer Res. 2018;8:1466–82.
Holland SJ, Pan A, Franci C, Hu Y, Chang B, Li W, et al. R428, a selective small molecule inhibitor of Axl kinase, blocks tumor spread and prolongs survival in models of metastatic breast cancer. Cancer Res. 2010;70:1544–54.
Loges S, Heuser M, Chromik J, Sutamtewagul G, Kapp-Schwoerer S, Crugnola M. et al. Phase Ib/II study (NCT02488408/BGBC003) of bemcentinib monotherapy or in combination with cytarabine or decitabine in acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS): FINAL results. Blood. 2023;142:4287.
Leconet W, Larbouret C, Chardès T, Thomas G, Neiveyans M, Busson M, et al. Preclinical validation of AXL receptor as a target for antibody-based pancreatic cancer immunotherapy. Oncogene. 2014;33:5405–14.
Chang H, An R, Li X, Lang X, Feng J, Lv M. Anti-Axl monoclonal antibodies attenuate the migration of MDA-MB-231 breast cancer cells. Oncol Lett. 2021;22:749.
Ye X, Li Y, Stawicki S, Couto S, Eastham-Anderson J, Kallop D, et al. An anti-Axl monoclonal antibody attenuates xenograft tumor growth and enhances the effect of multiple anticancer therapies. Oncogene. 2010;29:5254–64.
Leconet W, Chentouf M, du Manoir S, Chevalier C, Sirvent A, Ait-Arsa I, et al. Therapeutic activity of anti-AXL antibody against triple-negative breast cancer patient-derived xenografts and metastasis. Clin Cancer Res. 2017;23:2806–16.
Wei J, Sun H, Zhang A, Wu X, Li Y, Liu J, et al. A novel AXL chimeric antigen receptor endows T cells with anti-tumor effects against triple negative breast cancers. Cell Immunol. 2018;331:49–58.
Farschtschi S, Park SJ, Sawitzki B, Oh SJ, Kluwe L, Mautner VF, et al. Effector T cell subclasses associate with tumor burden in neurofibromatosis type 1 patients. Cancer Immunol Immunother. 2016;65:1113–21.
Landers SM, Bhalla AD, Ma X, Lusby K, Ingram D, Al Sannaa G, et al. AXL inhibition enhances MEK inhibitor sensitivity in malignant peripheral nerve sheath tumors. J Cancer Sci Clin Ther. 2020;4:511–25.
Sengsayadeth S, Savani BN, Oluwole O, Dholaria B. Overview of approved CAR-T therapies, ongoing clinical trials, and its impact on clinical practice. eJHaem. 2022;3:6–10.
Cho JH, Okuma A, Al-Rubaye D, Intisar E, Junghans RP, Wong WW. Engineering Axl specific CAR and SynNotch receptor for cancer therapy. Sci Rep. 2018;8:3846.
Cao B, Liu M, Wang L, Zhu K, Cai M, Chen X, et al. Remodelling of tumour microenvironment by microwave ablation potentiates immunotherapy of AXL-specific CAR T cells against non-small cell lung cancer. Nat Commun. 2022;13:6203.
Maher J, Davies DM. CAR-based immunotherapy of solid tumours—a survey of the emerging targets. Cancers. 2023;15:1171.
Hudecek M, Lupo-Stanghellini MT, Kosasih PL, Sommermeyer D, Jensen MC, Rader C, et al. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin Cancer Res. 2013;19:3153–64.
Lynn RC, Feng Y, Schutsky K, Poussin M, Kalota A, Dimitrov DS, et al. High-affinity FRβ-specific CAR T cells eradicate AML and normal myeloid lineage without HSC toxicity. Leukemia. 2016;30:1355–64.
Hombach AA, Schildgen V, Heuser C, Finnern R, Gilham DE, Abken H. T cell activation by antibody-like immunoreceptors: the position of the binding epitope within the target molecule determines the efficiency of activation of redirected T cells1. J Immunol. 2007;178:4650–7.
Robert B, Fauvel B, Cheve G, Yasri A, Larbouret C, Leconet W, et al. Inventor; Institut National de la Santé et de la Recherche Médicale; OriBase Pharma; Universitie de Montpellier 1; Centre Val d’Aurelle Paul Lamarque, assignee. Anti-AXL antibodies and uses thereof. US patent US 9.249,228 B2. 2012.
Mao R, Kong W, He Y. The affinity of antigen-binding domain on the antitumor efficacy of CAR T cells: moderate is better. Front Immunol. 2022;13:1032403.
Torres KC, Lima G, Simoes ESAC, Lubambo I, Rodrigues LO, Rodrigues L, et al. Immune markers in the RASopathy neurofibromatosis type 1. J Neuroimmunol. 2016;295-296:122–9.
Haworth KB, Arnold MA, Pierson CR, Choi K, Yeager ND, Ratner N, et al. Immune profiling of NF1-associated tumors reveals histologic subtype distinctions and heterogeneity: implications for immunotherapy. Oncotarget. 2017;8:47.
Tannenbaum C, Hamilton TA. Immune-inflammatory mechanisms in IFNgamma-mediated anti-tumor activity. Semin Cancer Biol. 2000;10 2:113–23.
Kiritsy MC, McCann K, Mott D, Holland SM, Behar SM, Sassetti CM, et al. Mitochondrial respiration contributes to the interferon gamma response in antigen-presenting cells. eLife. 2021;10:e65109.
Amitrano AM, Berry BJ, Lim K, Kim KD, Waugh RE, Wojtovich AP, et al. Optical control of CD8(+) T cell metabolism and effector functions. Front Immunol. 2021;12:666231.
Chen J, Chernatynskaya AV, Li J-W, Kimbrell MR, Cassidy RJ, Perry DJ, et al. T cells display mitochondria hyperpolarization in human type 1 diabetes. Sci Rep. 2017;7:10835.
Domingues S, Isidoro L, Rocha D, Sales Marques J. Neurofibromatosis type 1: a novel NF1 mutation associated with mitochondrial complex i deficiency. Case Rep Genet. 2014;2014:423071.
Allaway RJ, Wood MD, Downey SL, Bouley SJ, Traphagen NA, Wells JD, et al. Exploiting mitochondrial and metabolic homeostasis as a vulnerability in NF1 deficient cells. Oncotarget. 2018;9:15860–75.
Huang PY, Shih IA, Liao YC, You HL, Lee MJ. FT895 impairs mitochondrial function in malignant peripheral nerve sheath tumor cells. Int J Mol Sci. 2023;25:277.
Acknowledgements
Technique and facility support were provided by the third common laboratory at the National Taiwan University Hospital, Taipei, Taiwan. We thank the Academia Sinica Inflammation Core Facility (IBMS) for their technical support. The core facility was funded by the Academia Sinica Core Facility and Innovative Instrument Project (AS-CFII-111-213). We acknowledge Dr. Kai Wang for his critical reading and editing of the manuscript.
Funding
This research was funded by the National Science and Technology Council (NSTC 109-2314-B-002-121-MY3, 113-2314-B-002-275-MY3), Ministry of Education (112L7249, 113L7220), Executive Yuan, Taiwan, and National Taiwan University Hospital (NTUH 112-S257).
Author information
Authors and Affiliations
Contributions
P-YH and M-JL designed the study and developed the methodology. I-AS, Y-CL and H-LY acquired the data or helped with the data analysis. P-YH and M-JL discussed and interpreted the data. P-YH wrote the manuscript. C-TL and M-JL edited the manuscript. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
The NTUH Research Ethics Committee approved the study protocol (REC number: 202203085RIND). The study protocol was approved by the Institutional Animal Care and Use Committee of the National Taiwan University College of Medicine (IACUC number: 20220051). All methods were performed in accordance with the relevant guidelines and regulations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, PY., Shih, IA., Liao, YC. et al. Evaluating CAR-T cells from neurofibromatosis type 1 (NF1) patients for targeting AXL in malignant peripheral nerve sheath tumors associated with NF1. Br J Cancer 133, 1365–1376 (2025). https://doi.org/10.1038/s41416-025-03151-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41416-025-03151-w