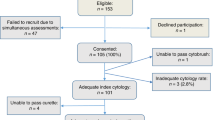
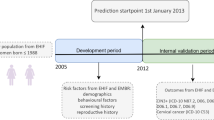
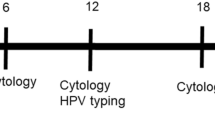
Abstract
The European Council recommends adopting risk-based screening when relevant. In triaging HPV-positive women, it can be an effective strategy to reduce overtreatment and referral to colposcopy. HPV genotyping and p16/ki67 expression may allow a better risk stratification than cytology. In Italy, recommendations on their use (alone or combined) in screening were developed by a multi-professional (nine scientific societies) and multidisciplinary working group (including patients and decision makers). Grading of Recommendations Assessment, Development and Evaluation (GRADE) Evidence to Decision frameworks were used. Data from large clinical trials on screening populations with long follow-up instructed the biomarkers’ evaluation. The working group defined the CIN3+ risk thresholds (a surrogate marker of cancer risk) to guide decisions on management: immediate colposcopy, referral to 1-year and 3-year retesting. The risk-based approach allowed to reduce the number of possible strategies to be compared to five specific healthcare questions framed as PICOs. The prioritised outcomes were risk of cancer and of CIN3+ in HPV+/triage-negative women, number of colposcopies, number of samples to be taken, and number of unneeded treatments. The combination of morphological markers (cytology or p16/ki67) and extended HPV genotyping was the only strategy with a conditional recommendation in favour when compared with cytology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The guideline development project and final recommendations were published on the database of the Italian National System of Guidelines (SNLG) of the National Institute of Health (ISS) (Sistema Nazionale Linee Guida, Istituto Superiore di Sanità). https://www.iss.it/-/snlg-prevenzione-carcinoma-cervice-uterina. It includes the complete Evidence-to-Decision and Summary of Findings tables (and relative references), and is available upon request to the corresponding author; simplified copies are included in the Supplementary material.
References
Ronco G, Confortini M, Maccallini V, Naldoni C, Segnan N, Sideri M, et al. Health Technology Assessment Report. Uso della citologia in fase liquida nello screening dei precursori del cancro del collo uterino [Health technology assessment report. Use of liquid-based cytology for cervical cancer precursors screening]. Epidemiol Prev. 2012;36:e1–33.
Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJ, Arbyn M, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524–32. https://doi.org/10.1016/S0140-6736(13)62218-7.
Confortini M, Giorgi Rossi P, Barbarino P, Passarelli AM, Orzella L, Tufi MC. Screening for cervical cancer with the human papillomavirus test in an area of central Italy with no previous active cytological screening programme. J Med Screen. 2010;17:79–86. https://doi.org/10.1258/jms.2010.009092
Zorzi M, Frayle H, Rizzi M, Fedato C, Rugge M, Penon MG, et al. A 3-year interval is too short for re-screening women testing negative for human papillomavirus: a population-based cohort study. BJOG Int J Obstet Gynaecol. 2017;124:1585–93. https://doi.org/10.1111/1471-0528.14575
Del Mistro A, Frayle H, Ferro A, Callegaro S, Del Sole A, Stomeo A, et al. Cervical cancer screening by high risk HPV testing in routine practice: results at one year recall of high risk HPV-positive and cytology-negative women. J Med Screen. 2014;21:30–7. https://doi.org/10.1177/0969141314522219
Pasquale L, Giorgi Rossi P, Carozzi F, Pedretti C, Ruggeri C, Scalvinoni V, et al. Cervical cancer screening with HPV testing in the Valcamonica (Italy) screening programme. J Med Screen. 2015;22:38–48. https://doi.org/10.1177/0969141314561707
Pasquale L, Giorgi Rossi P, Carozzi F, Domenighini S, Ruggeri C, Cecconami L, et al. HPV screening performance indicators in women who previously tested HPV-negative: the second round of Vallecamonica screening programme, Northern Italy. J Med Screen. 2020;27:207–14. https://doi.org/10.1177/0969141320905325
Passamonti BU, Bulletti S, Gustinucci D, Martinelli N, D’Amico MR, Spita N, et al. Cervical human papilloma virus (HPV) DNA primary screening test results of the experience of a regional laboratory in Central Italy. Value Health. 2014;17:A614. https://doi.org/10.1016/j.jval.2014.08.2162
Passamonti B, Gustinucci D, Giorgi Rossi P, Cesarini E, Bulletti S, Carlani A, et al. Cervical human papilloma virus (HPV) DNA primary screening test: results of a population-based screening programme in central Italy. J Med Screen. 2017;24:153–62. https://doi.org/10.1177/0969141316663580
Di Stefano F, Giorgi Rossi P, Carozzi F, Ronco G, Cacciani L, Vecchi S, et al. L’implementazione del DNA-HPV come test primario nei programmi italiani di screening del cervicocarcinoma. Risultati del Progetto MIDDIR [Implementation of DNA-HPV primary screening in Italian cervical cancer screening programmes. Results of the MIDDIR Project]. Epidemiol Prev. 2017;41:116–24. https://doi.org/10.19191/EP17.2.P116.031
Ronco G, Arbyn M, Meijer CJLM, Snijders PJF, Cuzick J. Screening for cervical cancer with primary testing for human papillomavirus. S1. In: Anttila A, Arbyn M, De Vuyst H, et al., editors. European guidelines for quality assurance in cervical cancer screening. 2nd edn, Supplements. Luxembourg: Office for Official Publications of the European Union; 2015. pp. 1–68. Available at: https://www.gisci.it/documenti/news/EW0115451ENN_002.pdf. Accessed 11 Nov 2024.
Giorgi Rossi P, Carozzi F, Ronco G, Allia E, Bisanzi S, Gillio-Tos A, et al. p16/ki67 and E6/E7 mRNA accuracy and prognostic value in triaging HPV DNA-positive women. J Natl Cancer Inst. 2021;113:292–300. https://doi.org/10.1093/jnci/djaa105
Gustinucci D, Benevolo M, Cesarini E, Mancuso P, Passamonti B, Giaimo MD, et al. Accuracy of different triage strategies for human papillomavirus positivity in an Italian screening population. Int J Cancer. 2022;150:952–60. https://doi.org/10.1002/ijc.33858
GISCi. Documento operativo Gisci per l’applicazione nei programmi di screening del sistema Bethesda 2001. Firenze, 2006. Available at: https://www.gisci.it/documenti/documenti_gisci/doc_operativo_bethesda.pdf. Accessed 11 Nov 2024.
Dalla Palma P, Giorgi Rossi P, Collina G, Buccoliero AM, Ghiringhello B, Lestani M, et al. The risk of false-positive histology according to the reason for colposcopy referral in cervical cancer screening: a blind revision of all histologic lesions found in the NTCC trial. Am J Clin Pathol. 2008;129:75–80. https://doi.org/10.1309/EWYGWFRRM8798U5P
Venturelli F, On Behalf Of The Multisociety Italian Guidelines For Cervical Cancer Prevention Working Group. Developing evidence-based Multisociety Italian Guidelines for cervical cancer prevention: rationale, methods, and development process. Eur J Gynaecol Oncol. 2021;42:634–42. https://doi.org/10.31083/j.ejgo4204098
Sistema Nazionale Linee guida – ISS. Linee guida condivise per la prevenzione del carcinoma della cervice uterina. GISCI-Gruppo Italiano Screening Cervicocarcinoma, AIO, AOGOI, SIAPEC-IAP, SICi, SICPCV, SIGO, SItI, SIV-ISV. 2024. Available at: https://www.iss.it/-/snlg-prevenzione-carcinoma-cervice-uterina. Accessed 11 Nov 2024.
Cuschieri K, Ronco G, Lorincz A, Smith L, Ogilvie G, Mirabello L, et al. Eurogin roadmap 2017: triage strategies for the management of HPV-positive women in cervical screening programs. Int J Cancer. 2018;143:735–45. https://doi.org/10.1002/ijc.31261
Taghavi K, Zhao F, Downham L, Baena A, Basu P. Molecular triaging options for women testing HPV positive with self-collected samples. Front Oncol. 2023;13:1243888. https://doi.org/10.3389/fonc.2023.1243888
Cuschieri K, Wentzensen N. Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia. Cancer Epidemiol Biomark Prev. 2008;17:2536–45. https://doi.org/10.1158/1055-9965.EPI-08-0306
Bergeron C, Ronco G, Reuschenbach M, Wentzensen N, Arbyn M, Stoler M, et al. The clinical impact of using p16(INK4a) immunochemistry in cervical histopathology and cytology: an update of recent developments. Int J Cancer. 2015;136:2741–51. https://doi.org/10.1002/ijc.28900
Wentzensen N, Sherman ME, Schiffman M, Wang SS. Utility of methylation markers in cervical cancer early detection: appraisal of the state-of-the-science. Gynecol Oncol. 2009;112:293–9. https://doi.org/10.1016/j.ygyno.2008.10.012
Giorgi Rossi P, Ronco G, Mancuso P, Carozzi F, Allia E, Bisanzi S, et al. Performance of HPV E6/E7 mRNA assay as primary screening test: results from the NTCC2 trial. Int J Cancer. 2022;151:1047–58. https://doi.org/10.1002/ijc.34120
Benevolo M, Ronco G, Mancuso P, Carozzi F, De Marco L, Allia E, et al. Comparison of HPV-positive triage strategies combining extended genotyping with cytology or p16/ki67 dual staining in the Italian NTCC2 study. EBioMedicine. 2024;104:105149 https://doi.org/10.1016/j.ebiom.2024.105149
Ronco G, Zappa M, Franceschi S, Tunesi S, Caprioglio A, Confortini M, et al. Impact of variations in triage cytology interpretation on human papillomavirus-based cervical screening and implications for screening algorithms. Eur J Cancer. 2016;68:148–55. https://doi.org/10.1016/j.ejca.2016.09.008
Perkins RB, Guido RS, Castle PE, Chelmow D, Einstein MH, Garcia F, et al. 2019 ASCCP risk-based management consensus guidelines: updates through 2023. J Low Genit Trac Dis. 2024;28:3–6. https://doi.org/10.1097/LGT.0000000000000788
Perkins RB, Guido RS, Castle PE, Chelmow D, Einstein MH, Garcia F, et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Trac Dis. 2020;24:102–31. https://doi.org/10.1097/LGT.0000000000000525
Egemen D, Perkins RB, Clarke MA, Guido R, Huh W, Saraiya M, et al. Risk-based cervical consensus guidelines: methods to determine management if less than 5 years of data are available. J Low Genit Trac Dis. 2022;26:195–201. https://doi.org/10.1097/LGT.0000000000000685
Bouvard V, Wentzensen N, Mackie A, Berkhof J, Brotherton J, Giorgi-Rossi P, et al. The IARC perspective on cervical cancer screening. N. Engl J Med. 2021;385:1908–18. https://doi.org/10.1056/NEJMsr2030640
Giorgi Rossi P, Iossa A, Visioli CB, Venturelli F, Garutti P. Development of evidence-based guidelines for follow up of women treated for cervical intraepithelial neoplasia grade 2 or 3 (CIN2/3) in Italian screening programmes. Eur J Gynaecol Oncol. 2021;42:1079–92. https://doi.org/10.31083/j.ejgo4205157
Sistema Nazionale Linee guida – ISS. Linee guida condivise per la prevenzione del carcinoma della cervice uterina. Linee guida - Prevenzione del carcinoma della cervice uterina: gestione delle donne in follow-up post trattamento per CIN2 e CIN3. GISCI-Gruppo Italiano Screening Cervicocarcinoma, AIO, AOGOI, SIAPEC-IAP, SICi, SICPCV, SIGO, SItI, SIV-ISV. 2021. Available at: https://www.iss.it/documents/20126/8403884/LLGG-197-GISCi-cervice-utero_14ott_Racc2.pdf/ee4a467f-dbb7-663c-e93f-188275c98217?t=1678805434381. Accessed 21 Nov 2024.
Sistema Nazionale Linee guida – ISS. Manuale metodologico per la produzione di linee guida di pratica clinica. SNLG – ISS v. 1.3.3 March 2023. 2023. Available at: https://www.iss.it/-/snlg-manuale-metodologico Accessed 21 Nov 2024.
Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64:380–2. https://doi.org/10.1016/j.jclinepi.2010.09.011
Ronco G, Giorgi-Rossi P, Carozzi F, Confortini M, Dalla Palma P, Del Mistro A, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249–57. https://doi.org/10.1016/S1470-2045(09)70360-2
COHEAHR (Comparing health services interventions for the prevention of HPV-related cancer) project funded by the European Union’s Seventh Framework Programme for research, technological development and demonstration under grant agreement no FP7-F3-2013-603019. (2013-2018) Final report. Available at: https://cordis.europa.eu/project/id/603019/reporting. Accessed 21 Nov 2024.
ONS. Rapporto sul 2022. Osservatorio Nazionale Screening. 2024. Available at: https://www.osservatorionazionalescreening.it/sites/default/files/allegati/Rapporto%20ONS%202022.pdf. Accessed 21 Nov 2024.
ISS. Rapporto sull’evento nascita in Italia (CeDAP) - anno 2022. Istituto Superiore di Sanità. 2023. Available at: https://www.epicentro.iss.it/materno/cedap-2022. Accessed 21 Nov 2024.
Kyrgiou M, Athanasiou A, Kalliala IEJ, Paraskevaidi M, Mitra A, Martin-Hirsch PP, et al. Obstetric outcomes after conservative treatment for cervical intraepithelial lesions and early invasive disease. Cochrane Database Syst Rev. 2017;11:CD012847 https://doi.org/10.1002/14651858.CD012847
Carozzi F, Gillio-Tos A, Confortini M, Del Mistro A, Sani C, De Marco L, et al. Risk of high-grade cervical intraepithelial neoplasia during follow-up in HPV-positive women according to baseline p16-INK4A results: a prospective analysis of a nested substudy of the NTCC randomised controlled trial. Lancet Oncol. 2013;14:168–76. https://doi.org/10.1016/S1470-2045(12)70529-6
Cuzick J, Adcock R, Carozzi F, Gillio-Tos A, De Marco L, Del Mistro A, et al. Combined use of cytology, p16 immunostaining and genotyping for triage of women positive for high-risk human papillomavirus at primary screening. Int J Cancer. 2020;147:1864–73. https://doi.org/10.1002/ijc.32973
Polman NJ, Snijders PJF, Kenter GG, Berkhof J, Meijer CJLM. HPV-based cervical screening: Rationale, expectations and future perspectives of the new Dutch screening programme. Prev Med;. 2019;119:108–17. https://doi.org/10.1016/j.ypmed.2018.12.021
IARC. Cervical cancer screening. In: IARC handbooks of cancer prevention vol. 18. pp. 1–456 (2022). Available from: https://publications.iarc.fr/604. Accessed 21 Nov 2024.
Clarke MA, Cheung LC, Castle PE, Schiffman M, Tokugawa D, Poitras N, et al. Five-year risk of cervical precancer following p16/Ki-67 dual-stain triage of HPV-positive women. JAMA Oncol. 2019;5:181–6. https://doi.org/10.1001/jamaoncol.2018.4270
Acknowledgements
We thank Paola Lippi for her valuable support.
Author information
Authors and Affiliations
Consortia
Contributions
TM, RG, PC, GN, AP, MB, FM, ET, FMB, PM, AI, PG (as Technical Scientific Committee members or additional collaborators at the start of the guidelines project) conceived the guidelines, defined the scope, and prioritised the Health Questions in each chapter. ADM, PGR, FC (as chair or co-chairs) defined the protocol for literature reviews and modelling. SG, FV (as Evidence Review Team members) conducted the research, extracted the data, and filled the Sof and EtD for plenary sessions. ADM, PGR, SG, FV, FC edited the draft recommendations after plenary sessions and made changes to take into account the reviewers’ comments. ADM cured the contacts with the Italian National guideline system, submitted and got approved the recommendations. EA, BA, KLA, PA, MB, SB, AB, SB, EB, FC, EC, LC, CC, LDM, PF, HF, CF, PG, DG, VM, LM, MM, SM, VN, BP, TR, CS, PSdB, MLT, EV, CBV (as panel members) participated in prioritisation of outcome and framing of PICOs, took an active part in the plenary sessions, voted or agreed to each criteria, reviewed the draft recommendations and approved the final version after review. LDM also conceived the reported chapter of the guidelines. IB, MZ (as external reviewers) revised the review and modelling protocol. IB, MZ, ADI, and EPP (as external reviewers) reviewed the draft of the recommendations, asking for integrations and clarifications. PGR, FV, ADM, and SG drafted and edited the manuscript; FC edited the manuscript. All contributed to the final version of the guidelines and fulfilled the criteria for contributors according to the ICMJE criteria. All the authors and contributors read and approved the final version of the manuscript and agreed with its submission to the journal. ADM, as corresponding author, confirms to have read the Nature journal policies on author responsibilities and is submitting the manuscript in accordance with those policies.
Corresponding author
Ethics declarations
Competing interests
SG, FV, FC, and ADM have disclosed no conflicts of interest; PGR participated in the preparation of the IARC Handbook 18 on cervical screening. COIs were collected from all the Contributor Group members. Overall, seven members disclosed potential conflicts of interest and these COIs are included in the final document published on the database of the Italian National System of Guidelines (SNLG) of the National Institute of Health (ISS) (Sistema Nazionale Linee Guida, Istituto Superiore di Sanità): https://www.iss.it/documents/20126/8403884/LG_197_GISCi_Biomarcatori-screening-cervicale_29ago24.pdf/40999ebc-246c-fe99-d483-4da375d9f76a?t=1724836991806. The Technical Scientific Committee members judged as “potentially relevant” those of three members; they were allowed full participation in the vote, with COI public disclosure.
Ethics approval and consent to participate
Not applicable. The guideline development project has been submitted to and approved by the Italian National System of Guidelines (SNLG) of the National Institute of Health (ISS) (Sistema Nazionale Linee Guida, Istituto Superiore di Sanità) https://snlg.iss.it/.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gori, S., Venturelli, F., Carozzi, F. et al. Italian guidelines for cervical cancer screening. Multisocietal recommendations on the use of biomarkers in HPV screening with risk-based approach and GRADE methodology. Br J Cancer (2025). https://doi.org/10.1038/s41416-025-03161-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41416-025-03161-8