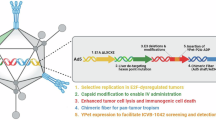
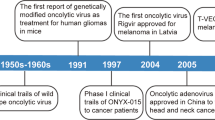
Abstract
The 11th International Oncolytic Virus Conference (IOVC) was held from April 9–12, 2018 in Oxford, UK. This is part of the high-profile academic-led series of meetings that was started back in 2002 by Steve Russell and John Bell, with most of the previous meetings being held in North America (often in Banff). The conference brought together many of the major players in oncolytic virotherapy from all over the world, addressing all stages of research and development—from aspects of basic science and cellular immunology all the way through to early- and late-phase clinical trials. The meeting welcomed 352 delegates from 24 countries. The top seven delegate countries, namely, the UK, US, Canada, The Netherlands, Germany, Japan and South Korea, contributed 291 delegates while smaller numbers coming from Australia, Austria, Bulgaria, China, Finland, France, Iraq, Ireland, Israel, Italy, Latvia, Malaysia, Poland, Slovenia, Spain, Sweden and Switzerland. Academics comprised about half of the attendees, industry 30% and students 20%. The next IOVC is scheduled to be held on Vancouver Island in autumn 2019. Here we share brief summaries of the oral presentations from invited speakers and proffered papers in the different subtopics presented at IOVC 2018.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Russell SJ, Federspiel MJ, Peng K-W, Tong C, Dingli D, Morice WG, et al. Remission of disseminated cancer after systemic oncolytic virotherapy. Mayo Clin Proc. 2014;89:926–33.
Cheng X, Wang W, Xu Q, Harper J, Carroll D, Galinski MS, et al. Genetic modification of oncolytic Newcastle disease virus for cancer therapy. J Virol. 2016;90:5343–52.
Muñoz-Alía MÁ, Muller CP, Russell SJ. Hemagglutinin-specific neutralization of subacute sclerosing panencephalitis viruses. PLoS ONE. 2018;13:e0192245.
Ammayappan A, Russell SJ, Federspiel MJ. Recombinant mumps virus as a cancer therapeutic agent. Mol Ther Oncolytics. 2016;3:16019.
Ribas A, Dummer R, Puzanov I, VanderWalde A, Andtbacka RHI, Michielin O, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2017;170:1109.e10–19.e10.
Streby KA, Geller JI, Currier MA, Warren PS, Racadio JM, Towbin AJ, et al. Intratumoral injection of HSV1716, an oncolytic herpes virus, is safe and shows evidence of immune response and viral replication in young cancer patients. Clin Cancer Res. 2017;23:3566–74.
Rodríguez-García A, Giménez-Alejandre M, Rojas JJ, Moreno R, Bazan-Peregrino M, Cascalló M, et al. Safety and efficacy of VCN-01, an oncolytic adenovirus combining fiber HSG-binding domain replacement with RGD and hyaluronidase expression. Clin Cancer Res. 2015;21:1406–18.
Zhang L, Steele MB, Jenks N, Grell J, Suksanpaisan L, Naik S, et al. Safety studies in tumor and non-tumor-bearing mice in support of clinical trials using oncolytic VSV-IFNβ-NIS. Hum Gene Ther Clin Dev. 2016;27:111–22.
Garcia-Carbonero R, Salazar R, Duran I, Osman-Garcia I, Paz-Ares L, Bozada JM, et al. Phase 1 study of intravenous administration of the chimeric adenovirus enadenotucirev in patients undergoing primary tumor resection. J Immunother Cancer. 2017;5:71.
Koodie L, Eidenschink B, Sell J, LaRocca C, Jacobsen K, Ryvlin J, et al. Effect of adenoviral death protein on NIS-based iodine therapy and imaging for pancreatic cancer. Pancreatology. 2016;16:S54.
Kim M, Nitschké M, Sennino B, Murer P, Schriver BJ, Bell A, et al. Amplification of oncolytic vaccinia virus widespread tumor cell killing by sunitinib through multiple mechanisms. Cancer Res. 2018;78:922–37.
Saha D, Martuza RL, Rabkin SD. Macrophage polarization contributes to glioblastoma eradication by combination immunovirotherapy and immune checkpoint blockade. Cancer Cell. 2017;32:253.e5–67.e5.
Cloughesy TF, Landolfi J, Hogan DJ, Bloomfield S, Carter B, Chen CC, et al. Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma. Sci Transl Med. 2016;8:341ra75
Mitchell LA, Lopez Espinoza F, Mendoza D, Kato Y, Inagaki A, Hiraoka K, et al. Toca 511 gene transfer and treatment with the prodrug, 5-fluorocytosine, promotes durable antitumor immunity in a mouse glioma model. Neuro Oncol. 2017;19:930–9.
Yagiz K, Rodriguez-Aguirre ME, Lopez Espinoza F, Montellano TT, Mendoza D, Mitchell LA, et al. A retroviral replicating vector encoding cytosine deaminase and 5-FC induces immune memory in metastatic colorectal cancer models. Mol Ther Oncolytics. 2018;8:14–26.
Lang FF, Conrad C, Gomez-Manzano C, Yung WKA, Sawaya R, Weinberg JS, et al. Phase I study of DNX-2401 (Delta-24-RGD) oncolytic adenovirus: replication and immunotherapeutic effects in recurrent malignant glioma. J Clin Oncol. 2018;36:1419–27.
Adair RA, Roulstone V, Scott KJ, Morgan R, Nuovo GJ, Fuller M, et al. Cell carriage, delivery, and selective replication of an oncolytic virus in tumor in patients. Sci Transl Med. 2012;4:138ra77.
Ilett E, Kottke T, Donnelly O, Thompson J, Willmon C, Diaz R, et al. Cytokine conditioning enhances systemic delivery and therapy of an oncolytic virus. Mol Ther. 2014;22:1851–63.
Parrish C, Scott GB, Migneco G, Scott KJ, Steele LP, Ilett E, et al. Oncolytic reovirus enhances rituximab-mediated antibody-dependent cellular cytotoxicity against chronic lymphocytic leukaemia. Leukemia. 2015;29:1799–810.
Gundem G, Van Loo P, Kremeyer B, Alexandrov LB, Tubio JMC, Papaemmanuil E, et al. The evolutionary history of lethal metastatic prostate cancer. Nature. 2015;520:353–7.
Cooper CS, Eeles R, Wedge DC, Van Loo P, Gundem G, Alexandrov LB, et al. Analysis of the genetic phylogeny of multifocal prostate cancer identifies multiple independent clonal expansions in neoplastic and morphologically normal prostate tissue. Nat Genet. 2015;47:367–72.
Speck T, Heidbuechel JPW, Veinalde R, Jaeger D, von Kalle C, Ball CR, et al. Targeted BiTE expression by an oncolytic vector augments therapeutic efficacy against solid tumors. Clin Cancer Res. 2018;24:2128–37.
Tan G, Kasuya H, Sahin TT, Yamamura K, Wu Z, Koide Y, et al. Combination therapy of oncolytic herpes simplex virus HF10 and bevacizumab against experimental model of human breast carcinoma xenograft. Int J Cancer. 2015;136:1718–30.
Eissa IR, Naoe Y, Bustos-Villalobos I, Ichinose T, Tanaka M, Zhiwen W, et al. Genomic signature of the natural oncolytic herpes simplex virus HF10 and its therapeutic role in preclinical and clinical trials. Front Oncol. 2017;7:149.
Yoshihiro H, Kasuya H, Bustos-Villalobos I, Naoe Y, Ichinose T, Tanaka M, et al. Curative effect of HF10 on liver and peritoneal metastasis mediated by host antitumor immunity. Oncolytic Virother. 2017;6:31–8.
Xia T, Konno H, Barber GN. Recurrent loss of STING signaling in melanoma correlates with susceptibility to viral oncolysis. Cancer Res. 2016;76:6747–59.
Ahn J, Konno H, Barber GN. Diverse roles of STING-dependent signaling on the development of cancer. Oncogene. 2015;34:5302–8.
Xia T, Konno H, Ahn J, Barber GN. Deregulation of STING signaling in colorectal carcinoma constrains DNA damage responses and correlates with tumorigenesis. Cell Rep. 2016;14:282–97.
Ahn J, Son S, Oliveira SC, Barber GN. STING-dependent signaling underlies IL-10 controlled inflammatory colitis. Cell Rep. 2017;21:3873–84.
Dai P, Wang W, Yang N, Serna-Tamayo C, Ricca JM, Zamarin D, et al. Intratumoral delivery of inactivated modified vaccinia virus Ankara (iMVA) induces systemic antitumor immunity via STING and Batf3-dependent dendritic cells. Sci Immunol. 2017;2:eaal1713.
Denton N, Chen C-Y, Scott T, Cripe T. Tumor-associated macrophages in oncolytic virotherapy: friend or foe? Biomedicines. 2016;4:13.
Bottermann M, James LC. Intracellular antiviral immunity. Adv Virus Res. 2018;100:309–54.
Watkinson RE, McEwan WA, Tam JCH, Vaysburd M, James LC. TRIM21 promotes cGAS and RIG-I sensing of viral genomes during infection by antibody-opsonized virus. PLoS Pathog. 2015;11:e1005253.
Dickson C, Fletcher AJ, Vaysburd M, Yang J-C, Mallery DL, Zeng J, et al. Intracellular antibody signalling is regulated by phosphorylation of the Fc receptor TRIM21. Elife. 2018;7:e32660
Urbaniak C, Gloor GB, Brackstone M, Scott L, Tangney M, Reid G. The microbiota of breast tissue and its association with breast cancer. Appl Environ Microbiol. 2016;82:5039–48.
Flores Bueso Y, Lehouritis P, Tangney M. In situ biomolecule production by bacteria; a synthetic biology approach to medicine. J Control Release. 2018;275:217–28.
Jiang S-N, Park S-H, Lee HJ, Zheng JH, Kim H-S, Bom H-S, et al. Engineering of bacteria for the visualization of targeted delivery of a cytolytic anticancer agent. Mol Ther. 2013;21:1985–95.
Felgner S, Kocijancic D, Frahm M, Weiss S. Bacteria in cancer therapy: renaissance of an old concept. Int J Microbiol. 2016;2016:1–14.
Felgner S, Kocijancic D, Pawar V, Weiss S. Biomimetic Salmonella: a next-generation therapeutic vector? Trends Microbiol. 2016;24:850–2.
Kocijancic D, Leschner S, Felgner S, Komoll R-M, Frahm M, Pawar V, et al. Therapeutic benefit of Salmonella attributed to LPS and TNF-α is exhaustible and dictated by tumor susceptibility. Oncotarget. 2017;8:36492–508.
Felgner S, Pawar V, Kocijancic D, Erhardt M, Weiss S. Tumour-targeting bacteria-based cancer therapies for increased specificity and improved outcome. Microb Biotechnol. 2017;10:1074–8.
Felgner S, Kocijancic D, Frahm M, Heise U, Rohde M, Zimmermann K, et al. Engineered Salmonella enterica serovar Typhimurium overcomes limitations of anti-bacterial immunity in bacteria-mediated tumor therapy. Oncoimmunology. 2018;7:e1382791.
Vincent D, Kramberger P, Hudej R, Štrancar A, Wang Y, Zhou Y, et al. The development of a monolith-based purification process for Orthopoxvirus vaccinia virus Lister strain. J Chromatogr A. 2017;1524:87–100.
Yuan M, Webb E, Lemoine N, Wang Y. CRISPR-Cas9 as a powerful tool for efficient creation of oncolytic viruses. Viruses. 2016;8:72.
Dyer A, Di Y, Calderon H, Illingworth S, Kueberuwa G, Tedcastle A, et al. Oncolytic group B adenovirus enadenotucirev mediates non-apoptotic cell death with membrane disruption and release of inflammatory mediators. Mol Ther Oncolytics. 2017;4:18–30.
Rajani K, Shim KG, Kazemi NY, Gendron W, Kottke T, Molan A, et al. 64. Generation of tumor cells resistant to oncolysis is mediated through virus induced APOBEC expression. Mol Ther. 2016;24:S28.
Colbeck EJ, Jones E, Hindley JP, Smart K, Schulz R, Browne M, et al. Treg depletion licenses T cell–driven HEV neogenesis and promotes tumor destruction. Cancer Immunol Res. 2017;5:1005–15.
Eriksson E, Milenova I, Wenthe J, Ståhle M, Leja-Jarblad J, Ullenhag G, et al. Shaping the tumor stroma and sparking immune activation by CD40 and 4-1BB signaling induced by an armed oncolytic virus. Clin Cancer Res. 2017;23:5846–57.
Wing A, Fajardo CA, Posey AD, Shaw C, Da T, Young RM, et al. Improving CART-cell therapy of solid tumors with oncolytic virus-driven production of a bispecific T-cell engager. Cancer Immunol Res. 2018;6:605–16.
Oh E, Choi I-K, Hong J, Yun C-O. Oncolytic adenovirus coexpressing interleukin-12 and decorin overcomes Treg-mediated immunosuppression inducing potent antitumor effects in a weakly immunogenic tumor model. Oncotarget. 2017;8:4730–46.
Ahn HM, Hong JW, Yun C-O. Oncolytic adenovirus coexpressing interleukin-12 and shVEGF restores antitumor immune function and enhances antitumor efficacy. Oncotarget. 2016;7:84965–80.
Freedman JD, Hagel J, Scott EM, Psallidas I, Gupta A, Spiers L, et al. Oncolytic adenovirus expressing bispecific antibody targets T‐cell cytotoxicity in cancer biopsies. EMBO Mol Med. 2017;9:1067–87.
Mell LK, Brumund KT, Daniels GA, Advani SJ, Zakeri K, Wright ME, et al. Phase I trial of intravenous oncolytic vaccinia virus (GL-ONC1) with cisplatin and radiotherapy in patients with locoregionally advanced head and neck carcinoma. Clin Cancer Res. 2017;23:5696–702.
Tsoneva D, Minev B, Frentzen A, Zhang Q, Wege AK, Szalay AA. Humanized mice with subcutaneous human solid tumors for immune response analysis of vaccinia virus-mediated oncolysis. Mol Ther Oncolytics. 2017;5:41–61.
Lilly CL, Villa NY, Lemos de Matos A, Ali HM, Dhillon J-KS, Hofland T, et al. Ex vivo oncolytic virotherapy with myxoma virus arms multiple allogeneic bone marrow transplant leukocytes to enhance graft versus tumor. Mol Ther Oncolytics. 2017;4:31–40.
AL-Janabi H, Conner J, Taher Z, Staniland S, Muthana M. Breast cancer immunotherapy using magnetised oncolytic viruses. Eur J Cancer. 2018;92:S4.
Myers R, Coviello C, Erbs P, Foloppe J, Rowe C, Kwan J, et al. Polymeric cups for cavitation-mediated delivery of oncolytic vaccinia virus. Mol Ther. 2016;24:1627–33.
Kwan JJ, Myers R, Coviello CM, Graham SM, Shah AR, Stride E, et al. Ultrasound-propelled nanocups for drug delivery. Small. 2015;11:5305–14.
Myers R, Grundy M, Rowe C, Coviello C, Bau L, Erbs P, et al. Ultrasound-mediated cavitation does not decrease the activity of small molecule, antibody or viral-based medicines. Int J Nanomed. 2018;ume 13:337–49.
Hiraoka K, Inagaki A, Kato Y, Huang TT, Mitchell LA, Kamijima S, et al. Retroviral replicating vector-mediated gene therapy achieves long-term control of tumor recurrence and leads to durable anticancer immunity. Neuro Oncol. 2017;19:918–29.
Marchini A, Bonifati S, Scott EM, Angelova AL, Rommelaere J. Oncolytic parvoviruses: from basic virology to clinical applications. Virol J. 2015;12:6.
Del Papa J, Parks RJ. Adenoviral vectors armed with cell fusion-inducing proteins as anti-cancer agents. Viruses. 2017;9:13
Wilkinson MJ, Smith HG, Pencavel TD, Mansfield DC, Kyula-Currie J, Khan AA, et al. Isolated limb perfusion with biochemotherapy and oncolytic virotherapy combines with radiotherapy and surgery to overcome treatment resistance in an animal model of extremity soft tissue sarcoma. Int J Cancer. 2016;139:1414–22.
Wilkinson MJ, Smith HG, McEntee G, Kyula-Currie J, Pencavel TD, Mansfield DC, et al. Oncolytic vaccinia virus combined with radiotherapy induces apoptotic cell death in sarcoma cells by down-regulating the inhibitors of apoptosis. Oncotarget. 2016;7:81208–22.
Acknowledgements
We are deeply indebted to our sponsors: PsiOxus Therapeutics, Amgen, Oxford Genetics, Sartorius Stedim Biotech, Sillajen, the American Society Gene and Cell Therapy, Cancer Research UK, Replimune, StemImmune, Turnstone Biologics, Vyriad, AstraZeneca, Ignite Immunotherapy, Novasep, Tocagen, The Brain Tumour Charity, Cancer Research UK Oxford Centre, Imanis Life Science, MedImmune, Merck, Viralytics, DNAtrix, and VCN Biosciences. Sponsors played no role in programme design or abstract selection. All decisions were made by the IOVC organising committee.
Funding
The authors are supported by Cancer Research UK grant C552/A17720, Newton-Ungku Omar Fund grant MR/P012795/1, the Engineering and Physical Science Research Council (EPSRC) Doctoral Training Centre in Synthetic Biology, Medical Research Council (MRC) Doctoral Training Partnership, The Brain Tumour Charity, Oxford Institute of Radiation Oncology and Linacre College, Oxford.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
LWS holds equity in PsiOxus Therapeutics Ltd. All the other authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Dyer, A., Baugh, R., Chia, S.L. et al. Turning cold tumours hot: oncolytic virotherapy gets up close and personal with other therapeutics at the 11th Oncolytic Virus Conference. Cancer Gene Ther 26, 59–73 (2019). https://doi.org/10.1038/s41417-018-0042-1
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41417-018-0042-1
This article is cited by
-
First-in-human study of TK-positive oncolytic vaccinia virus delivered by adipose stromal vascular fraction cells
Journal of Translational Medicine (2019)